Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome
- PMID: 25845659
- PMCID: PMC4512228
- DOI: 10.2337/db14-1916
Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome
Abstract
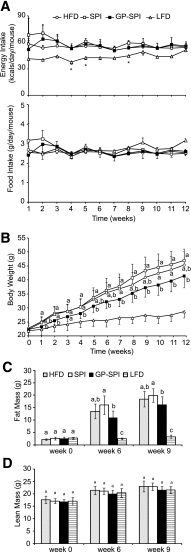
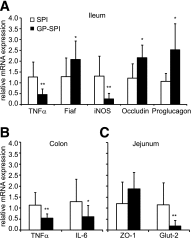
Dietary polyphenols protect against metabolic syndrome, despite limited absorption and digestion, raising questions about their mechanism of action. We hypothesized that one mechanism may involve the gut microbiota. To test this hypothesis, C57BL/6J mice were fed a high-fat diet (HFD) containing 1% Concord grape polyphenols (GP). Relative to vehicle controls, GP attenuated several effects of HFD feeding, including weight gain, adiposity, serum inflammatory markers (tumor necrosis factor [TNF]α, interleukin [IL]-6, and lipopolysaccharide), and glucose intolerance. GP lowered intestinal expression of inflammatory markers (TNFα, IL-6, inducible nitric oxide synthase) and a gene for glucose absorption (Glut2) VSports手机版. GP increased intestinal expression of genes involved in barrier function (occludin) and limiting triglyceride storage (fasting-induced adipocyte factor). GP also increased intestinal gene expression of proglucagon, a precursor of proteins that promote insulin production and gut barrier integrity. 16S rRNA gene sequencing and quantitative PCR of cecal and fecal samples demonstrated that GP dramatically increased the growth of Akkermansia muciniphila and decreased the proportion of Firmicutes to Bacteroidetes, consistent with prior reports that similar changes in microbial community structure can protect from diet-induced obesity and metabolic disease. These data suggest that GP act in the intestine to modify gut microbial community structure, resulting in lower intestinal and systemic inflammation and improved metabolic outcomes. The gut microbiota may thus provide the missing link in the mechanism of action of poorly absorbed dietary polyphenols. .
© 2015 by the American Diabetes Association. Readers may use this article as long as the work is properly cited, the use is educational and not for profit, and the work is not altered. V体育安卓版.
Figures
Comment in
-
Commentary: Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome.Front Immunol. 2017 Jul 27;8:850. doi: 10.3389/fimmu.2017.00850. eCollection 2017. Front Immunol. 2017. PMID: 28798745 Free PMC article. No abstract available.
References
-
- Wilson PWF, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005;112:3066–3072 - PubMed
-
- Cani PD, Osto M, Geurts L, Everard A. Involvement of gut microbiota in the development of low-grade inflammation and type 2 diabetes associated with obesity. Gut Microbes 2012;3:279–288 - VSports在线直播 - PMC - PubMed
Publication types
- V体育安卓版 - Actions
MeSH terms
- Actions (V体育平台登录)
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- "VSports手机版" Actions
- VSports - Actions
- "VSports" Actions
- "V体育官网入口" Actions
- V体育安卓版 - Actions
- VSports app下载 - Actions
- VSports最新版本 - Actions
Substances
- V体育2025版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
VSports最新版本 - Other Literature Sources
Medical
Molecular Biology Databases

