Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation
- PMID: 25792600
- PMCID: PMC4378197
- DOI: 10.1101/gad.256628.114
Brg1 promotes both tumor-suppressive and oncogenic activities at distinct stages of pancreatic cancer formation
Abstract
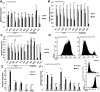
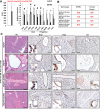
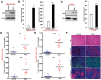
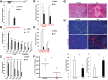
Pancreatic ductal adenocarcinoma (PDA) develops predominantly through pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN) precursor lesions VSports手机版. Pancreatic acinar cells are reprogrammed to a "ductal-like" state during PanIN-PDA formation. Here, we demonstrate a parallel mechanism operative in mature duct cells during which functional cells undergo "ductal retrogression" to form IPMN-PDA. We further identify critical antagonistic roles for Brahma-related gene 1 (Brg1), a catalytic subunit of the SWI/SNF complexes, during IPMN-PDA development. In mature duct cells, Brg1 inhibits the dedifferentiation that precedes neoplastic transformation, thus attenuating tumor initiation. In contrast, Brg1 promotes tumorigenesis in full-blown PDA by supporting a mesenchymal-like transcriptional landscape. We further show that JQ1, a drug that is currently being tested in clinical trials for hematological malignancies, impairs PDA tumorigenesis by both mimicking some and inhibiting other Brg1-mediated functions. In summary, our study demonstrates the context-dependent roles of Brg1 and points to potential therapeutic treatment options based on epigenetic regulation in PDA. .
Keywords: Brg1; EMT; IPMN; Kras; dedifferentiation; pancreatic cancer. V体育安卓版.
© 2015 Roy et al. ; Published by Cold Spring Harbor Laboratory Press V体育ios版. .
"V体育安卓版" Figures



Comment in
-
VSports - Pancreatic cancer: A matter of timing.Nat Rev Cancer. 2015 May;15(5):256-7. doi: 10.1038/nrc3948. Epub 2015 Apr 9. Nat Rev Cancer. 2015. PMID: 25855403 No abstract available.
References
-
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, et al.2006. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev 20: 3130–3146. - PMC (V体育ios版) - PubMed
-
- Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardiere C, et al.2011. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 364: 1817–1825. - PubMed
-
- Dal Molin M, Hong SM, Hebbar S, Sharma R, Scrimieri F, de Wilde RF, Mayo SC, Goggins M, Wolfgang CL, Schulick RD, et al.2012. Loss of expression of the SWI/SNF chromatin remodeling subunit BRG1/SMARCA4 is frequently observed in intraductal papillary mucinous neoplasms of the pancreas. Hum Pathol 43: 585–591. - PMC - PubMed
"V体育2025版" Publication types
"VSports" MeSH terms
- VSports手机版 - Actions
- V体育官网入口 - Actions
- VSports - Actions
- "VSports最新版本" Actions
- "VSports在线直播" Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
- "V体育ios版" Actions
- VSports app下载 - Actions
- V体育2025版 - Actions
- Actions (VSports app下载)
Substances
- V体育ios版 - Actions
- V体育平台登录 - Actions
- "V体育官网" Actions
- Actions (VSports在线直播)
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育2025版)
Medical
Molecular Biology Databases
Miscellaneous
