"V体育官网入口" ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer
- PMID: 25753158
- PMCID: PMC4423074
- DOI: V体育2025版 - 10.1038/cr.2015.30
ERK kinase phosphorylates and destabilizes the tumor suppressor FBW7 in pancreatic cancer
Abstract
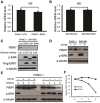
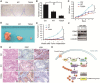
F-box and WD repeat domain-containing 7 (FBW7) is the substrate recognition component of the Skp1-Cul1-F-box (SCF) ubiquitin ligase complex and functions as a major tumor suppressor by targeting various oncoproteins for degradation VSports手机版. Genomic deletion or mutation of FBW7 has frequently been identified in many human cancers but not in pancreatic ductal adenocarcinoma. Thus it is important to know how the tumor suppressive function of FBW7 is impaired in pancreatic cancer. In this study, we first observed that low FBW7 expression correlated significantly with ERK activation in pancreatic cancer clinical samples, primarily due to KRAS mutations in pancreatic cancer. We further showed that ERK directly interacted with FBW7 and phosphorylated FBW7 at Thr205, which sequentially promoted FBW7 ubiquitination and proteasomal degradation. Furthermore, the phospho-deficient T205A FBW7 mutant is resistant to ERK activation and could significantly suppress pancreatic cancer cell proliferation and tumorigenesis. These results collectively demonstrate how the oncogenic KRAS mutation inhibits the tumor suppressor FBW7, thus revealing an important function of KRAS mutations in promoting pancreatic cancer progression. .
Figures

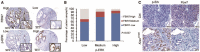



References
-
- Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. - PubMed
Publication types
MeSH terms (V体育2025版)
- "VSports最新版本" Actions
- V体育官网 - Actions
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- V体育ios版 - Actions
- "V体育平台登录" Actions
- VSports - Actions
- Actions (VSports在线直播)
- "VSports app下载" Actions
- "VSports在线直播" Actions
- "VSports注册入口" Actions
- Actions (VSports注册入口)
- "V体育安卓版" Actions
- VSports app下载 - Actions
- "V体育2025版" Actions
VSports手机版 - Substances
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- Actions (V体育官网)
- "VSports注册入口" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
VSports在线直播 - Medical
Molecular Biology Databases
Research Materials (V体育ios版)
Miscellaneous

