FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification
- PMID: 25720964
- PMCID: PMC4401662
- DOI: 10.1038/ncb3121
FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification
Abstract
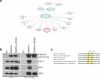
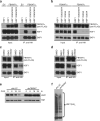
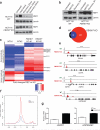
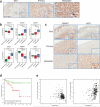
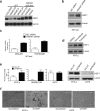
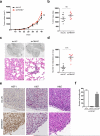
Heat-shock factor 1 (HSF1) orchestrates the heat-shock response in eukaryotes. Although this pathway has evolved to help cells adapt in the presence of challenging conditions, it is co-opted in cancer to support malignancy. However, the mechanisms that regulate HSF1 and thus cellular stress response are poorly understood. Here we show that the ubiquitin ligase FBXW7α interacts with HSF1 through a conserved motif phosphorylated by GSK3β and ERK1 VSports手机版. FBXW7α ubiquitylates HSF1 and loss of FBXW7α results in impaired degradation of nuclear HSF1 and defective heat-shock response attenuation. FBXW7α is either mutated or transcriptionally downregulated in melanoma and HSF1 nuclear stabilization correlates with increased metastatic potential and disease progression. FBXW7α deficiency and subsequent HSF1 accumulation activates an invasion-supportive transcriptional program and enhances the metastatic potential of human melanoma cells. These findings identify a post-translational mechanism of regulation of the HSF1 transcriptional program both in the presence of exogenous stress and in cancer. .
Figures (VSports在线直播)

References
-
- Pelham HR. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982;30:517–528. - V体育官网 - PubMed
-
- Westerheide SD, Morimoto RI. Heat shock response modulators as therapeutic tools for diseases of protein conformation. The Journal of biological chemistry. 2005;280:33097–33100. - PubMed
-
- Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nature reviews. Cancer. 2005;5:761–772. - PubMed
-
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. Journal of the National Cancer Institute. 2000;92:1564–1572. - PubMed
-
- Jin X, Moskophidis D, Mivechi NF. Heat shock transcription factor 1 is a key determinant of HCC development by regulating hepatic steatosis and metabolic syndrome. Cell metabolism. 2011;14:91–103. - V体育官网 - PMC - PubMed
Publication types
- "V体育安卓版" Actions
- "VSports手机版" Actions
"VSports" MeSH terms
- VSports app下载 - Actions
- Actions (V体育安卓版)
- "VSports app下载" Actions
- VSports app下载 - Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
- "VSports" Actions
- Actions (VSports在线直播)
- Actions (VSports app下载)
- VSports手机版 - Actions
- VSports app下载 - Actions
- Actions (VSports app下载)
- VSports app下载 - Actions
- "VSports" Actions
- V体育官网 - Actions
- "VSports最新版本" Actions
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- VSports手机版 - Actions
- Actions (VSports最新版本)
- "V体育官网" Actions
- V体育平台登录 - Actions
- Actions (VSports)
Substances
- Actions (V体育平台登录)
- "VSports" Actions
- "VSports" Actions
- "V体育ios版" Actions
- Actions (VSports注册入口)
- Actions (V体育官网)
- Actions (V体育2025版)
- VSports最新版本 - Actions
- V体育官网入口 - Actions
Grants and funding
- 1R01CA169784-02/CA/NCI NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- R01 CA133379/CA/NCI NIH HHS/United States
- R01 CA093471/CA/NCI NIH HHS/United States
- V体育官网入口 - R01 CA202027/CA/NCI NIH HHS/United States
- R01 CA149655/CA/NCI NIH HHS/United States
- V体育官网 - R01 CA105129/CA/NCI NIH HHS/United States
- VSports注册入口 - R21 GM110450/GM/NIGMS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 CA169784/CA/NCI NIH HHS/United States
- R01 CA163891/CA/NCI NIH HHS/United States
- R24 OD018339/OD/NIH HHS/United States
- 1R01CA1638-01A1/CA/NCI NIH HHS/United States
- 1R01CA105129-08/CA/NCI NIH HHS/United States
- 5R01CA173636-03/CA/NCI NIH HHS/United States
- V体育平台登录 - 1R01CA149655-04/CA/NCI NIH HHS/United States
- VSports - R01 CA173636/CA/NCI NIH HHS/United States
- 1R01CA155234/CA/NCI NIH HHS/United States
- 1R01CA133379-06/CA/NCI NIH HHS/United States
- 1R24OD018339-01/OD/NIH HHS/United States
- R01 CA155234/CA/NCI NIH HHS/United States
LinkOut - more resources
"VSports最新版本" Full Text Sources
Other Literature Sources
VSports最新版本 - Medical
Molecular Biology Databases
Miscellaneous

