HIV-1 Tat induces DNMT over-expression through microRNA dysregulation in HIV-related non Hodgkin lymphomas
- PMID: 25705251
- PMCID: PMC4334912
- DOI: 10.1186/1750-9378-9-41
"V体育ios版" HIV-1 Tat induces DNMT over-expression through microRNA dysregulation in HIV-related non Hodgkin lymphomas
Abstract
Background: A close association between HIV infection and the development of cancer exists VSports手机版. Although the advent of highly active antiretroviral therapy has changed the epidemiology of AIDS-associated malignancies, a better understanding on how HIV can induce malignant transformation will help the development of novel therapeutic agents. .
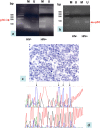
Methods: HIV has been reported to induce the expression of DNMT1 in vitro, but still no information is available about the mechanisms regulating DNMT expression in HIV-related B-cell lymphomas. In this paper, we investigated the expression of DNMT family members (DNMT1, DNMT3a/b) in primary cases of aggressive B-cell lymphomas of HIV-positive subjects. V体育安卓版.
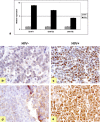
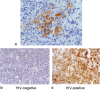
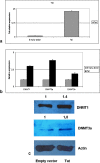
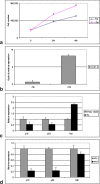
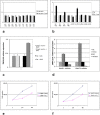
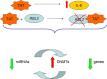
Results: Our results confirmed the activation of DNMT1 by HIV in vivo, and reported for the first time a marked up-regulation of DNMT3a and DNMT3b in HIV-positive aggressive B-cell lymphomas. DNMT up-regulation in HIV-positive tumors correlated with down-regulation of specific microRNAs, as the miR29 family, the miR148-152 cluster, known to regulate their expression V体育ios版. Literature reports the activation of DNMTs by the human polyomavirus BKV large T-antigen and adenovirus E1a, through the pRb/E2F pathway. We have previously demonstrated that the HIV Tat protein is able to bind to the pocket proteins and to inactivate their oncosuppressive properties, resulting in uncontrolled cell proliferation. Therefore, we focused on the role of Tat, due to its capability to be released from infected cells and to dysregulate uninfected ones, using an in vitro model in which Tat was ectopically expressed in B-cells. .
Conclusions: Our findings demonstrated that the ectopic expression of Tat was per se sufficient to determine DNMT up-regulation, based on microRNA down-regulation, and that this results in aberrant hypermethylation of target genes and microRNAs. These results point at a direct role for Tat in participating in uninfected B-cell lymphomagenesis, through dysregulation of the epigenetical control of gene expression VSports最新版本. .
Keywords: Aggressive B-cell lymphomas; DNMTs; HIV; Tat; microRNAs V体育平台登录. .
Figures


References
-
- World Health Organization Classification of Tumours . In: Tumours of Haematopoietic and Lymphoid tissues. Jaffe ES, Harris NL, Stein H, Vardimar JW, editors. Lyon: IARC Press; 2008.
-
- Moir S, Fauci A. Pathogenic mechanisms of B-lymphocyte dysfunction in HIV disease. J Allergy Clin Immunol. 2008;122:12–19. doi: 10.1016/j.jaci.2008.04.034. - "VSports注册入口" DOI - PMC - PubMed
-
- Moir S, Malaspina A, Ogwaro KM, Donoghue ET, Hallahan CW, Ehler LA, Liu S, Adelsberger J, Lapointe R, Hwu P, Baseler M, Orenstein JM, Chun TW, Mican JA, Fauci AS. HIV-1 induces phenotypic and functional perturbations of B cells in chronically infected individuals. Proc Natl Acad Sci. 2001;98:10362–10367. doi: 10.1073/pnas.181347898. - DOI - PMC - PubMed
-
- Moir S, Malaspina A, Pickeral OK, Donoghue ET, Vasquez J, Miller NJ, Krishnan SR, Planta MA, Turney JF, Justement JS, Kottilil S, Dybul M, Mican JM, Kovacs C, Chun TW, Birse CE, Fauci AS. Decreased survival of B cells of HIV-viremic patients mediated by altered expression of receptors of the TNF superfamily. J Exp Med. 2004;200:587–599. doi: 10.1084/jem.20032236. - DOI - PubMed
-
- Titanji K, De Milito A, Cagigi A, Thorstensson R, Grützmeier S, Atlas A, Hejdeman B, Kroon FP, Lopalco L, Nilsson A, Chiodi F. Loss of memory B cells impairs maintenance of long-term serologic memory during HIV-1 infection. Blood. 2006;108:1580–1587. doi: 10.1182/blood-2005-11-013383. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

