"VSports手机版" Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis
- PMID: 25638205
- PMCID: PMC4371881 (VSports)
- DOI: 10.1186/s12967-015-0417-0
"V体育安卓版" Exosomes released from human induced pluripotent stem cells-derived MSCs facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis
Abstract
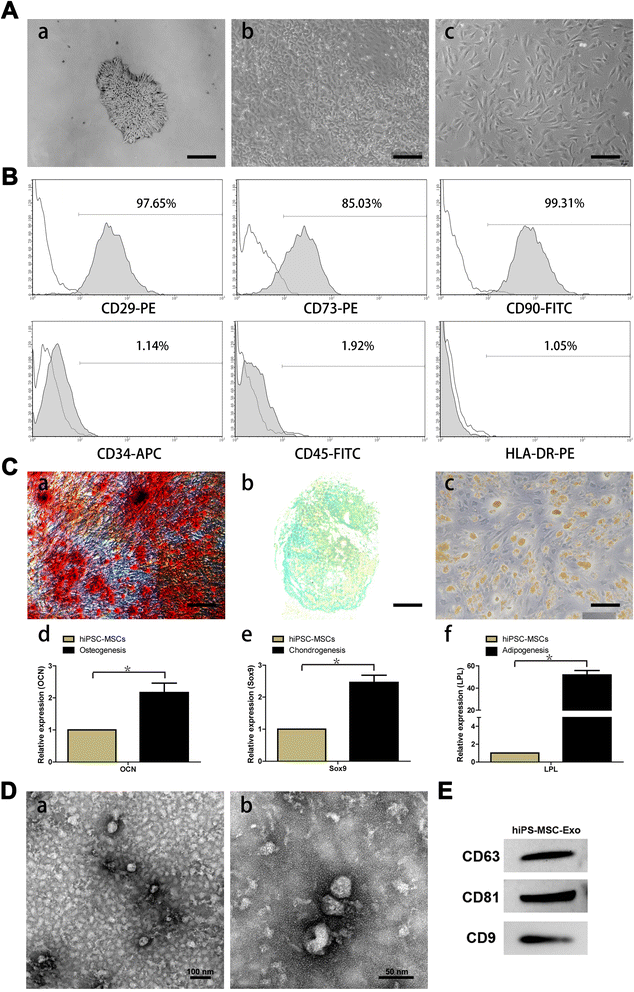
Background: Human induced pluripotent stem cell-derived mesenchymal stem cells (hiPSC-MSCs) have emerged as a promising alternative for stem cell transplantation therapy. Exosomes derived from mesenchymal stem cells (MSC-Exos) can play important roles in repairing injured tissues. However, to date, no reports have demonstrated the use of hiPSC-MSC-Exos in cutaneous wound healing, and little is known regarding their underlying mechanisms in tissue repair VSports手机版. .
Methods: hiPSC-MSC-Exos were injected subcutaneously around wound sites in a rat model and the efficacy of hiPSC-MSC-Exos was assessed by measuring wound closure areas, by histological and immunofluorescence examinations V体育安卓版. We also evaluated the in vitro effects of hiPSC-MSC-Exos on both the proliferation and migration of human dermal fibroblasts and human umbilical vein endothelial cells (HUVECs) by cell-counting and scratch assays, respectively. The effects of exosomes on fibroblast collagen and elastin secretion were studied in enzyme-linked immunosorbent assays and quantitative reverse-transcriptase-polymerase chain reaction (qRT-PCR). In vitro capillary network formation was determined in tube-formation assays. .
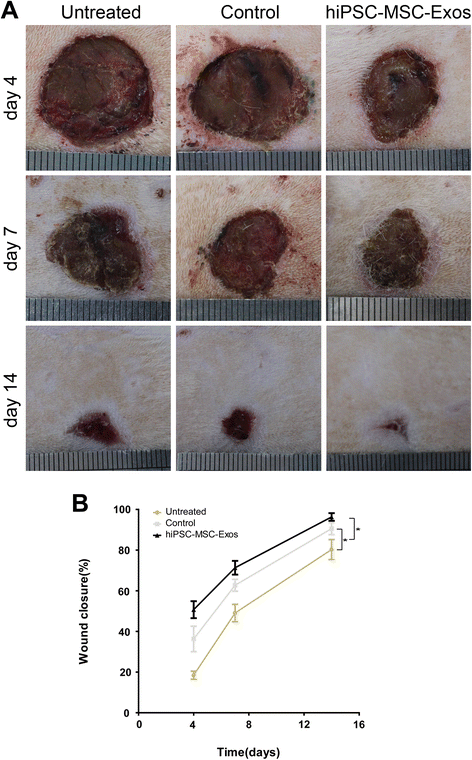
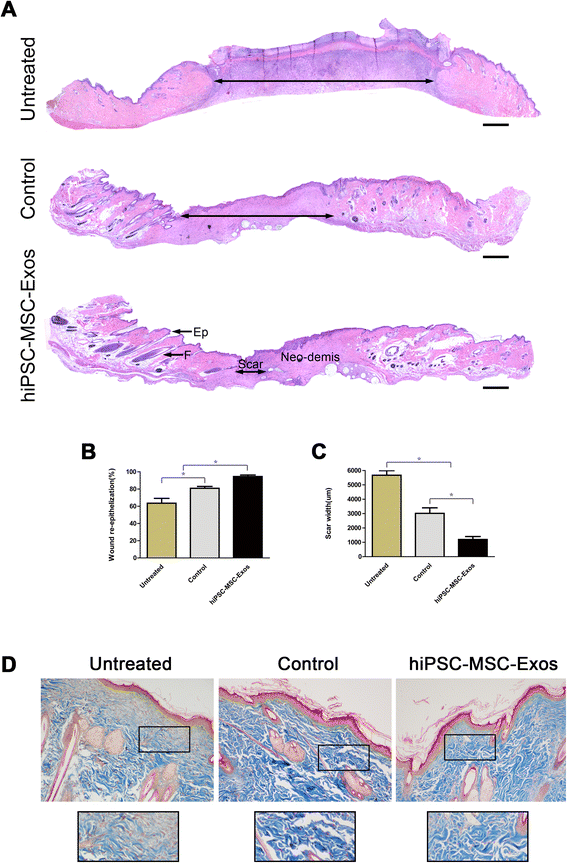
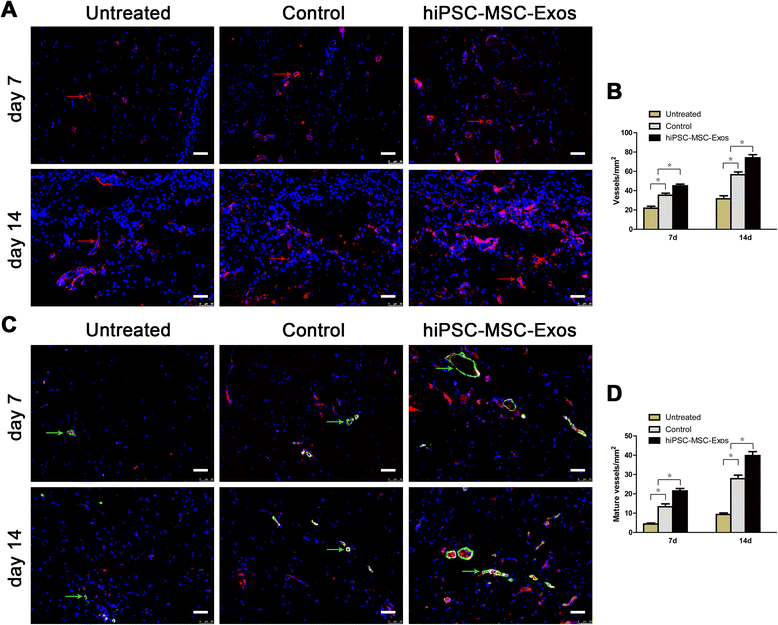
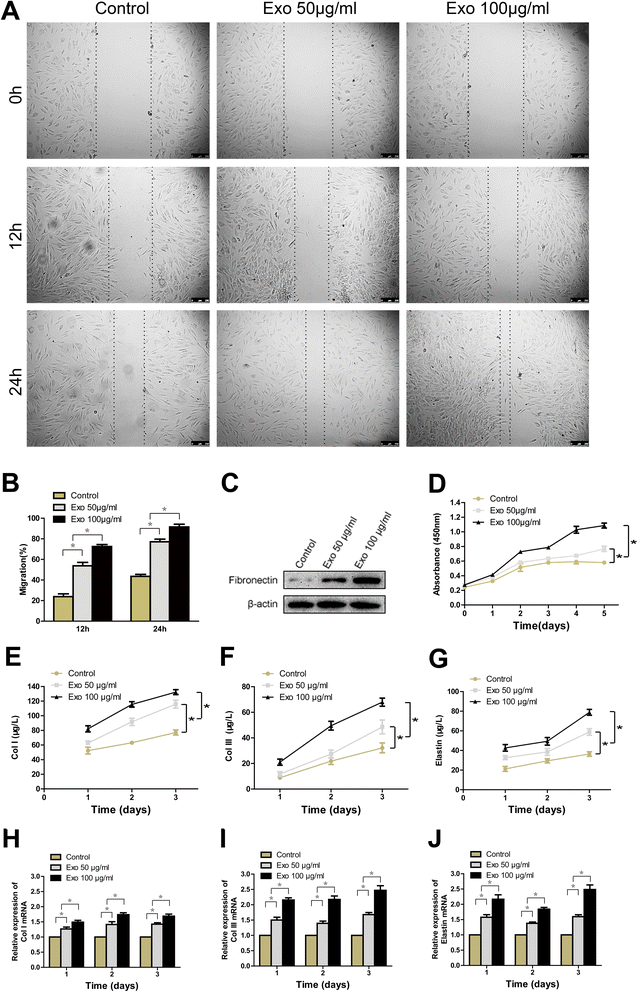
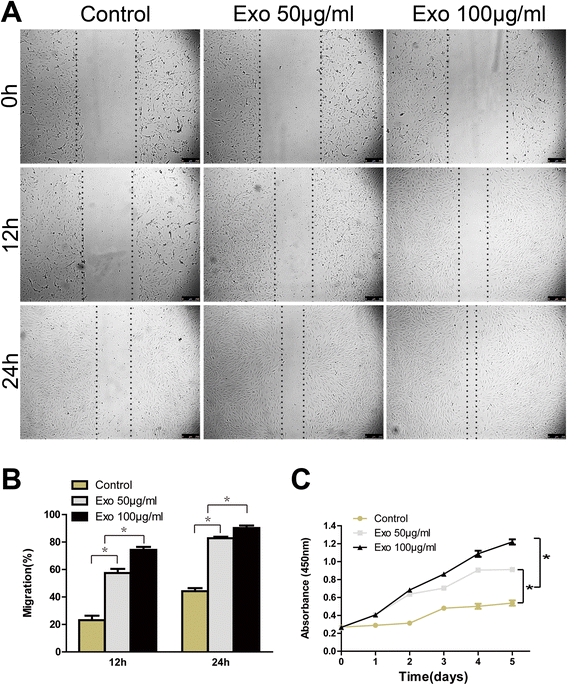
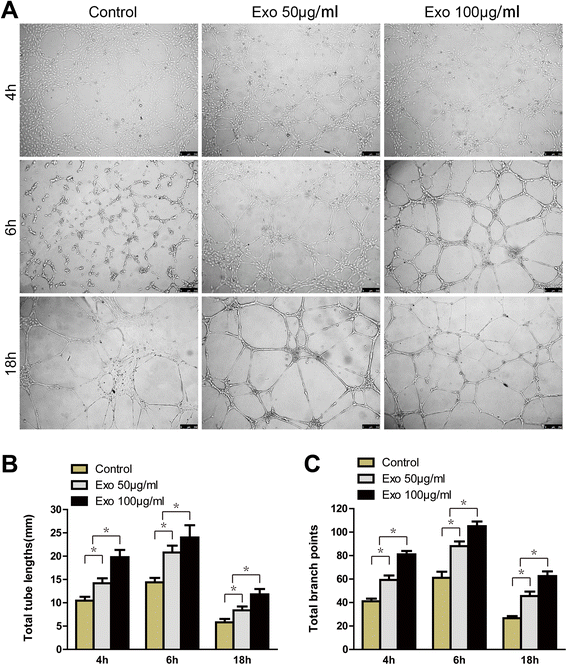
Results: Transplanting hiPSC-MSC-Exos to wound sites resulted in accelerated re-epithelialization, reduced scar widths, and the promotion of collagen maturity V体育ios版. Moreover, hiPSC-MSC-Exos not only promoted the generation of newly formed vessels, but also accelerated their maturation in wound sites. We found that hiPSC-MSC-Exos stimulated the proliferation and migration of human dermal fibroblasts and HUVECs in a dose-dependent manner in vitro. Similarly, Type I, III collagen and elastin secretion and mRNA expression by fibroblasts and tube formation by HUVECs were also increased with increasing hiPSC-MSC-Exos concentrations. .
Conclusions: Our findings suggest that hiPSC-MSC-Exos can facilitate cutaneous wound healing by promoting collagen synthesis and angiogenesis. These data provide the first evidence for the potential of hiPSC-MSC-Exos in treating cutaneous wounds VSports最新版本. .
Figures
References
Publication types
- V体育2025版 - Actions
MeSH terms
- VSports - Actions
- "VSports app下载" Actions
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- Actions (V体育官网)
- Actions (V体育官网)
- "V体育官网入口" Actions
- VSports app下载 - Actions
- Actions (V体育ios版)
- "VSports注册入口" Actions
- "V体育官网" Actions
- V体育2025版 - Actions
- V体育平台登录 - Actions
- V体育官网入口 - Actions
"VSports app下载" Substances
"VSports手机版" LinkOut - more resources
Full Text Sources (V体育官网入口)
Other Literature Sources
Medical

