Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice
- PMID: 25613133
- PMCID: PMC4416414
- DOI: "VSports" 10.2174/1570162x13666150121111548
VSports最新版本 - Didehydro-cortistatin A inhibits HIV-1 Tat mediated neuroinflammation and prevents potentiation of cocaine reward in Tat transgenic mice
Abstract
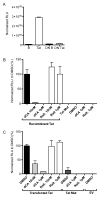
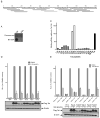
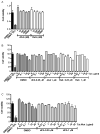
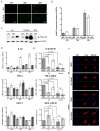
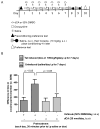
HIV-1 Tat protein has been shown to have a crucial role in HIV-1-associated neurocognitive disorders (HAND), which includes a group of syndromes ranging from undetectable neurocognitive impairment to dementia. The abuse of psychostimulants, such as cocaine, by HIV infected individuals, may accelerate and intensify neurological damage. On the other hand, exposure to Tat potentiates cocaine-mediated reward mechanisms, which further promotes HAND. Here, we show that didehydro-Cortistatin A (dCA), an analog of a natural steroidal alkaloid, crosses the blood-brain barrier, cross-neutralizes Tat activity from several HIV-1 clades and decreases Tat uptake by glial cell lines. In addition, dCA potently inhibits Tat mediated dysregulation of IL-1β, TNF-α and MCP-1, key neuroinflammatory signaling proteins. Importantly, using a mouse model where doxycycline induces Tat expression, we demonstrate that dCA reverses the potentiation of cocaine-mediated reward VSports手机版. Our results suggest that adding a Tat inhibitor, such as dCA, to current antiretroviral therapy may reduce HIV-1-related neuropathogenesis. .
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kaul M, Zheng J, Okamoto S, et al. HIV-1 infection and AIDS: consequences for the central nervous system. Cell Death Differ. 2005;12(Suppl 1):878–92. - PubMed
-
- Bagashev A, Sawaya BE. Roles and functions of HIV-1 Tat protein in the CNS: an overview. Virol J. 2013;10:358. - PMC (V体育安卓版) - PubMed
-
- Gonzalez-Scarano F, Martin-Garcia J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5(1):69–81. - PubMed
-
- Gorry PR, Howard JL, Churchill MJ, et al. Diminished production of human immunodeficiency virus type 1 in astrocytes results from inefficient translation of gag, env, and nef mRNAs despite efficient expression of Tat and Rev. J Virol. 1999;73(1):352–61. - "VSports手机版" PMC - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- VSports手机版 - Actions
- "VSports app下载" Actions
- V体育官网 - Actions
- V体育安卓版 - Actions
- Actions (VSports在线直播)
- V体育安卓版 - Actions
- Actions (V体育官网入口)
Substances
- Actions (VSports注册入口)
- Actions (V体育官网入口)
- VSports最新版本 - Actions
- VSports注册入口 - Actions
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
VSports app下载 - Research Materials
Miscellaneous
