VSports在线直播 - Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer
- PMID: 25605928
- PMCID: PMC4321320
- DOI: 10.1073/pnas.1424024112
Improved tumor vascularization after anti-VEGF therapy with carboplatin and nab-paclitaxel associates with survival in lung cancer
Abstract
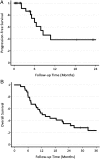
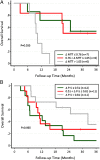
Addition of anti-VEGF antibody therapy to standard chemotherapies has improved survival and is an accepted standard of care for advanced non-small cell lung cancer (NSCLC). However, the mechanisms by which anti-VEGF therapy increases survival remain unclear. We evaluated dynamic CT-based vascular parameters and plasma cytokines after bevacizumab alone and after bevacizumab plus chemotherapy with carboplatin and nab-paclitaxel in advanced NSCLC patients to explore potential biomarkers of treatment response and resistance to this regimen. Thirty-six patients were enrolled in this study. The primary end point was 6-mo progression-free survival rate, which was 74% (95% CI: 57, 97). This regimen has a promising overall response rate of 36% and median time to progression of 8. 5 (6. 0, 38. 7) mo and overall survival of 12 VSports手机版. 2 (9. 6, 44. 1) mo. We found that anti-VEGF therapy led to a sustained increase in plasma PlGF, a potential pharmacodynamic marker. We also found that higher levels of soluble VEGFR1 measured before starting bevacizumab with chemotherapy were associated with worse survival, supporting its potential role as biomarker of treatment resistance. Our imaging biomarker studies indicate that bevacizumab-based treatment-while reducing blood flow, volume, and permeability in the overall population-may be associated with improved survival in patients with improved tumor vasculature and blood perfusion after treatment. This hypothesis-generating study supports the notion that excessively decreasing vascular permeability and pruning/rarefaction after bevacizumab therapy may negatively impact the outcome of combination therapy in NSCLC patients. This hypothesis warrants further dose-titration studies of bevacizumab to examine the dose effect on tumor vasculature and treatment efficacy. .
Keywords: antiangiogenesis; bioimaging; lung cancer V体育安卓版. .
Conflict of interest statement
Conflict of interest statement: R. S. H. has served as a consultant for Boehringer-Ingelheim and Momenta. D V体育ios版. G. D. has served as a consultant for Hexal/Sandoz. P. F. has served on the speakers’ bureau for Boehringer-Ingelheim and Genentech/Roche, and on an advisory board for Boehringer-Ingelheim and Genentech/Roche. L. V. S. has served as a consultant (uncompensated) for Clovis, Astrazeneca, Boehringer Ingelheim, Genentech/Roche, Novartis, Merrimack, and Taiho. J. S. T. received travel funds from Helsinn Therapeutics. A. T. S. has served as a consultant for Pfizer, Novartis, Ariad, Chugai, Ignyta, Genentech/Roche, and Daiichi-Sankyo. L. G. has served as a consultant for Merck and on an advisory board for Merck and Genentech/Roche. N. A. P. has served as a consultant for Genentech. J. W. N. has served as a consultant for Clovis. T. J. L. has served on the board of directors for BMS, on a scientific advisory board for Arvinas, and has an EGFR mutation-testing patent from Partners Healthcare. J. A. E. has received consulting fees from Genentech, Roche, Ventana, and Chugai, and is coinventor on a patent licensed by Ventana. R. K. J. has received research grants from MedImmune and Roche for research unrelated to this study; has received consultant fees from Enlight, Ophthotech, and SynDevRx; owns equity in Enlight, Ophthotech, SynDevRx, and XTuit; and serves on the Board of Directors of XTuit and Boards of Trustees of Tekla Healthcare Investors, Tekla Life Sciences Investors, and Tekla Healthcare Opportunities Fund. Funding for the clinical trial was provided by Celgene and Roche/Genentech, which provided study drugs and funding for the clinical trial. All data collection and analysis were performed independently by the investigators of this investigator-initiated clinical trial.
"VSports手机版" Figures
Comment in
-
Improving efficacy of the combination between antiangiogenic and chemotherapy: Time for mathematical modeling support.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3453. doi: 10.1073/pnas.1506689112. Epub 2015 Jun 16. Proc Natl Acad Sci U S A. 2015. PMID: 26080431 Free PMC article. No abstract available.
-
VSports手机版 - Reply to Ciccolini et al.: Using mathematical modeling to predict response to antiangiogenic therapy in cancer patients.Proc Natl Acad Sci U S A. 2015 Jul 7;112(27):E3454. doi: 10.1073/pnas.1507225112. Epub 2015 Jun 16. Proc Natl Acad Sci U S A. 2015. PMID: 26080434 Free PMC article. No abstract available.
References
-
- Sandler A, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542–2550. - PubMed
-
- Garon EB, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): A multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665–673. - PubMed
-
- Goel S, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev. 2011;91(3):1071–1121. - "V体育2025版" PMC - PubMed
"V体育ios版" Publication types
MeSH terms
- Actions (V体育ios版)
- "VSports注册入口" Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- V体育平台登录 - Actions
- "V体育平台登录" Actions
Substances
- Actions (V体育官网入口)
- Actions (V体育2025版)
- "VSports注册入口" Actions
- VSports - Actions
- VSports app下载 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

