A requirement for Gch1 and tetrahydrobiopterin in embryonic development
- PMID: 25557619
- PMCID: PMC4347993
- DOI: 10.1016/j.ydbio.2014.12.025
A requirement for Gch1 and tetrahydrobiopterin in embryonic development
Abstract
Introduction: GTP cyclohydrolase I (GTPCH) catalyses the first and rate-limiting reaction in the synthesis of the enzymatic cofactor, tetrahydrobiopterin (BH4). Loss of function mutations in the GCH1 gene lead to congenital neurological diseases such as DOPA-responsive dystonia and hyperphenylalaninemia VSports手机版. However, little is known about how GTPCH and BH4 affects embryonic development in utero, and in particular whether metabolic replacement or supplementation in pregnancy is sufficient to rescue genetic GTPCH deficiency in the developing embryo. .
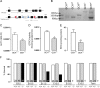
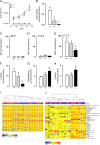
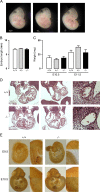
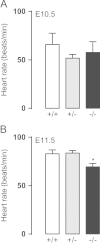
Methods and results: Gch1 deficient mice were generated by the insertion of loxP sites flanking exons 2-3 of the Gch1 gene. Gch1(fl/fl) mice were bred with Sox2cre mice to generate mice with global Gch1 deficiency V体育安卓版. Genetic ablation of Gch1 caused embryonic lethality by E13. 5. Despite loss of Gch1 mRNA and GTPCH enzymatic activity, whole embryo BH4 levels were maintained until E11. 5, indicating sufficient maternal transfer of BH4 to reach this stage of development. After E11. 5, Gch1(-/-) embryos were deficient in BH4, but an unbiased metabolomic screen indicated that the lethality was not due to a gross disturbance in metabolic profile. Embryonic lethality in Gch1(-/-) embryos was not caused by structural abnormalities, but was associated with significant bradycardia at E11. 5. Embryonic lethality was not rescued by maternal supplementation of BH4, but was partially rescued, up to E15. 5, by maternal supplementation of BH4 and l-DOPA. .
Conclusion: These findings demonstrate a requirement for Gch1 in embryonic development and have important implications for the understanding of pathogenesis and treatment of genetic BH4 deficiencies, as well as the identification of new potential roles for BH4. V体育ios版.
Keywords: Development; Embryo; GCH; Heart; Tetrahydrobiopterin VSports最新版本. .
Copyright © 2015 The Authors. Published by Elsevier Inc V体育平台登录. All rights reserved. .
Figures

References
-
- Brüggemann N., Spiegler J., Hellenbroich Y. BEneficial prenatal levodopa therapy in autosomal recessive guanosine triphosphate cyclohydrolase 1 deficiency. Arch. Neurol. 2012;69:1071–1075. - VSports注册入口 - PubMed
-
- Cosentino F., Barker J.E., Brand M.P., Heales S.J., Werner E.R., Tippins J.R., West N., Channon K.M., Volpe M., Luscher T.F. Reactive oxygen species mediate endothelium-dependent relaxations in tetrahydrobiopterin-deficient mice. Arterioscler Thromb. Vasc. Biol. 2001;21:496–502. - VSports手机版 - PubMed
-
- Costigan M., Latremoliere A., Woolf C.J. Analgesia by inhibiting tetrahydrobiopterin synthesis. Curr. Opin. Pharmacol. 2012;12:92–99. - "VSports注册入口" PMC - PubMed
-
- Cotton R.G.H. A model for hyperphenylalaninaemia due to tetrahydrobiopterin deficiency. J. Inherit. Metab. Dis. 1986;9:4–14. - PubMed
Publication types
- Actions (VSports在线直播)
MeSH terms
- V体育2025版 - Actions
- "VSports最新版本" Actions
- VSports手机版 - Actions
- VSports手机版 - Actions
- VSports app下载 - Actions
- "V体育官网" Actions
- "V体育安卓版" Actions
- Actions (V体育平台登录)
Substances
- VSports最新版本 - Actions
Grants and funding
- RG/12/5/29576/BHF_/British Heart Foundation/United Kingdom
- RG/10/15/28578/BHF_/British Heart Foundation/United Kingdom
- "V体育ios版" FS/14/56/31049/BHF_/British Heart Foundation/United Kingdom
- PG/15/35/31403/BHF_/British Heart Foundation/United Kingdom
- 07/003/23133/BHF_/British Heart Foundation/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

