Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production
- PMID: 25525874
- PMCID: PMC4520319 (VSports注册入口)
- DOI: 10.1016/j.cell.2014.11.036
"VSports注册入口" Apoptotic caspases suppress mtDNA-induced STING-mediated type I IFN production
Abstract
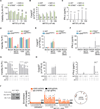
Activated caspases are a hallmark of apoptosis induced by the intrinsic pathway, but they are dispensable for cell death and the apoptotic clearance of cells in vivo. This has led to the suggestion that caspases are activated not just to kill but to prevent dying cells from triggering a host immune response VSports手机版. Here, we show that the caspase cascade suppresses type I interferon (IFN) production by cells undergoing Bak/Bax-mediated apoptosis. Bak and Bax trigger the release of mitochondrial DNA. This is recognized by the cGAS/STING-dependent DNA sensing pathway, which initiates IFN production. Activated caspases attenuate this response. Pharmacological caspase inhibition or genetic deletion of caspase-9, Apaf-1, or caspase-3/7 causes dying cells to secrete IFN-β. In vivo, this precipitates an elevation in IFN-β levels and consequent hematopoietic stem cell dysfunction, which is corrected by loss of Bak and Bax. Thus, the apoptotic caspase cascade functions to render mitochondrial apoptosis immunologically silent. .
Copyright © 2014 Elsevier Inc. All rights reserved. V体育安卓版.
Figures
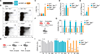

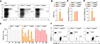
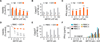

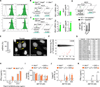
V体育官网入口 - Comment in
-
Cell death. Silencing the immune response of apoptotic cells.Nat Rev Immunol. 2015 Feb;15(2):68-9. doi: 10.1038/nri3808. Epub 2015 Jan 19. Nat Rev Immunol. 2015. PMID: 25598532
References
-
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. - PubMed
-
- Barber GN. STING-dependent cytosolic DNA sensing pathways. Trends Immunol. 2014;35:88–93. - PubMed
-
- Burguillos MA, Deierborg T, Kavanagh E, Persson A, Hajji N, Garcia-Quintanilla A, Cano J, Brundin P, Englund E, Venero JL, Joseph B. Caspase signalling controls microglia activation and neurotoxicity. Nature. 2011;472:319–324. - PubMed
-
- Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. - PubMed
Publication types
- "VSports手机版" Actions
V体育平台登录 - MeSH terms
- "VSports注册入口" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- V体育官网 - Actions
- "VSports" Actions
- Actions (VSports)
- V体育官网 - Actions
- V体育2025版 - Actions
Substances
- Actions (V体育安卓版)
- Actions (VSports最新版本)
- Actions (V体育ios版)
- V体育ios版 - Actions
- V体育2025版 - Actions
Associated data
- Actions
- VSports - Actions
Grants and funding
LinkOut - more resources
VSports最新版本 - Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

