Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia
- PMID: 25517747
- PMCID: "V体育官网入口" PMC4269830
- DOI: 10.1016/j.ccell.2014.10.006
Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia
Abstract
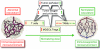
Ten antiangiogenic drugs targeting VEGF or its receptors are approved for cancer treatment. However, these agents, intended to block tumors' blood supply, may cause hypoxia, which may fuel tumor progression and treatment resistance. Emerging clinical data suggest that patients whose tumor perfusion or oxygenation increases in response to these agents may actually survive longer. Hence, strategies aimed at alleviating tumor hypoxia while improving perfusion may enhance the outcome of radiotherapy, chemotherapy, and immunotherapy VSports手机版. Here I summarize lessons learned from preclinical and clinical studies over the past decade and propose strategies for improving antiangiogenic therapy outcomes for malignant and nonmalignant diseases. .
Copyright © 2014 Elsevier Inc. All rights reserved. V体育安卓版.
Figures



References
-
- Aguilera KY, Rivera LB, Hur H, Carbon JG, Toombs JE, Goldstein CD, Dellinger MT, Castrillon DH, Brekken RA. Collagen Signaling Enhances Tumor Progression after Anti-VEGF Therapy in a Murine Model of Pancreatic Ductal Adenocarcinoma. Cancer Res. 2014;74:1032–1044. - "VSports最新版本" PMC - PubMed
-
- Arjaans M, Oude Munnink TH, Oosting SF, Terwisscha van Scheltinga AG, Gietema JA, Garbacik ET, Timmer-Bosscha H, Lub-de Hooge MN, Schroder CP, de Vries EG. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 2013;73:3347–3355. - PubMed (V体育官网入口)
-
- Barsoum IB, Smallwood CA, Siemens DR, Graham CH. A Mechanism of Hypoxia-Mediated Escape from Adaptive Immunity in Cancer Cells. Cancer Res. 2014;74:665–674. - PubMed
Publication types
- Actions (VSports手机版)
- VSports注册入口 - Actions
VSports注册入口 - MeSH terms
- Actions (V体育平台登录)
- VSports手机版 - Actions
- VSports - Actions
- Actions (VSports在线直播)
- "VSports手机版" Actions
Substances
VSports注册入口 - Grants and funding
LinkOut - more resources
Full Text Sources
"V体育ios版" Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

