"V体育官网" p53 Mutation Directs AURKA Overexpression via miR-25 and FBXW7 in Prostatic Small Cell Neuroendocrine Carcinoma
- PMID: 25512615
- PMCID: VSports在线直播 - PMC4369163
- DOI: 10.1158/1541-7786.MCR-14-0277-T
"V体育ios版" p53 Mutation Directs AURKA Overexpression via miR-25 and FBXW7 in Prostatic Small Cell Neuroendocrine Carcinoma
Abstract
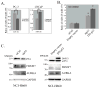
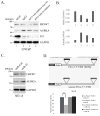
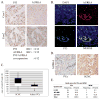
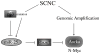
Prostatic small cell neuroendocrine carcinoma (SCNC) is a rare but aggressive form of prostate cancer that is negative for androgen receptor (AR) and not responsive to hormonal therapy VSports手机版. The molecular etiology of this prostate cancer variant is not well understood; however, mutation of the p53 (TP53) tumor suppressor in prostate neuroendocrine cells inactivates the IL8-CXCR2-p53 pathway that normally inhibits cellular proliferation, leading to the development of SCNC. SCNC also overexpresses Aurora kinase A (AURKA) which is considered to be a viable therapeutic target. Therefore, the relationship of these two molecular events was studied, and we show that p53 mutation leads to increased expression of miR-25 and downregulation of the E3 ubiquitin ligase FBXW7, resulting in elevated levels of Aurora kinase A. This study demonstrates an intracellular pathway by which p53 mutation leads to Aurora kinase A expression, which is critically important for the rapid proliferation and aggressive behavior of prostatic SCNC. .
Implications: The pathogenesis of prostatic SCNC involves a p53 and Aurora Kinase A signaling mechanism, both potentially targetable pathways. V体育安卓版.
©2014 American Association for Cancer Research V体育ios版. .
Conflict of interest statement
Figures

References (V体育ios版)
-
- Abrahamsson PA, Falkmer S, Falt K, Grimelius L. The course of neuroendocrine differentiation in prostatic carcinomas. An immunohistochemical study testing chromogranin A as an “endocrine marker”. Pathology, research and practice. 1989;185:373–80. - PubMed
-
- Abrahamsson PA, Wadstrom LB, Alumets J, Falkmer S, Grimelius L. Peptide-hormone- and serotonin-immunoreactive tumour cells in carcinoma of the prostate. Pathology, research and practice. 1987;182:298–307. - PubMed
-
- Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer discovery. 2011;1:487–95. - "VSports手机版" PMC - PubMed
-
- Zhang XQ, Kondrikov D, Yuan TC, Lin FF, Hansen J, Lin MF. Receptor protein tyrosine phosphatase alpha signaling is involved in androgen depletion-induced neuroendocrine differentiation of androgen-sensitive LNCaP human prostate cancer cells. Oncogene. 2003;22:6704–16. - PubMed
Publication types
- VSports注册入口 - Actions
MeSH terms
- V体育2025版 - Actions
- VSports - Actions
- "V体育官网" Actions
- "VSports" Actions
- "V体育平台登录" Actions
- "V体育官网入口" Actions
- "V体育安卓版" Actions
- Actions (VSports在线直播)
- "VSports最新版本" Actions
- V体育2025版 - Actions
- "V体育ios版" Actions
- "VSports" Actions
Substances (V体育2025版)
- Actions (V体育官网入口)
- Actions (V体育官网)
- V体育ios版 - Actions
- Actions (V体育平台登录)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

