Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function
- PMID: 25404286
- PMCID: PMC4260594
- DOI: 10.1073/pnas.1415756111
Caspase-1 autoproteolysis is differentially required for NLRP1b and NLRP3 inflammasome function
Abstract
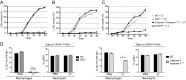
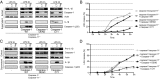
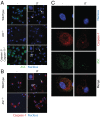
Inflammasomes are caspase-1-activating multiprotein complexes. The mouse nucleotide-binding domain and leucine rich repeat pyrin containing 1b (NLRP1b) inflammasome was identified as the sensor of Bacillus anthracis lethal toxin (LT) in mouse macrophages from sensitive strains such as BALB/c. Upon exposure to LT, the NLRP1b inflammasome activates caspase-1 to produce mature IL-1β and induce pyroptosis. Both processes are believed to depend on autoproteolysed caspase-1. In contrast to human NLRP1, mouse NLRP1b lacks an N-terminal pyrin domain (PYD), indicating that the assembly of the NLRP1b inflammasome does not require the adaptor apoptosis-associated speck-like protein containing a CARD (ASC). LT-induced NLRP1b inflammasome activation was shown to be impaired upon inhibition of potassium efflux, which is known to play a major role in NLRP3 inflammasome formation and ASC dimerization. We investigated whether NLRP3 and/or ASC were required for caspase-1 activation upon LT stimulation in the BALB/c background. The NLRP1b inflammasome activation was assessed in both macrophages and dendritic cells lacking either ASC or NLRP3. Upon LT treatment, the absence of NLRP3 did not alter the NLRP1b inflammasome activity VSports手机版. Surprisingly, the absence of ASC resulted in IL-1β cleavage and pyroptosis, despite the absence of caspase-1 autoprocessing activity. By reconstituting caspase-1/caspase-11(-/-) cells with a noncleavable or catalytically inactive mutant version of caspase-1, we directly demonstrated that noncleavable caspase-1 is fully active in response to the NLRP1b activator LT, whereas it is nonfunctional in response to the NLRP3 activator nigericin. Taken together, these results establish variable requirements for caspase-1 cleavage depending on the pathogen and the responding NLR. .
Keywords: dendritic cell; interleukin-1beta; lethal toxin; macrophage; pyroptosis V体育安卓版. .
Conflict of interest statement
The authors declare no conflict of interest.
V体育ios版 - Figures


VSports手机版 - References
-
- Banks DJ, Ward SC, Bradley KA. New insights into the functions of anthrax toxin. Expert Rev Mol Med. 2006;8(7):1–18. - PubMed
-
- Bradley KA, Mogridge J, Mourez M, Collier RJ, Young JA. Identification of the cellular receptor for anthrax toxin. Nature. 2001;414(6860):225–229. - PubMed
-
- Moayeri M, Leppla SH. The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol. 2004;7(1):19–24. - PubMed
-
- Alileche A, Serfass ER, Muehlbauer SM, Porcelli SA, Brojatsch J. Anthrax lethal toxin-mediated killing of human and murine dendritic cells impairs the adaptive immune response. PLoS Pathog. 2005;1(2):e19. - "V体育官网入口" PMC - PubMed
-
- Agrawal A, et al. Impairment of dendritic cells and adaptive immunity by anthrax lethal toxin. Nature. 2003;424(6946):329–334. - PubMed
Publication types
- VSports在线直播 - Actions
MeSH terms
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- Actions (V体育ios版)
- "V体育2025版" Actions
- VSports注册入口 - Actions
- VSports - Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- VSports注册入口 - Actions
- "VSports" Actions
- Actions (VSports注册入口)
- V体育ios版 - Actions
- "VSports手机版" Actions
- Actions (V体育2025版)
- Actions (V体育平台登录)
Substances
- VSports在线直播 - Actions
- Actions (VSports注册入口)
- Actions (V体育安卓版)
- VSports - Actions
- "VSports注册入口" Actions
- Actions (V体育ios版)
- "V体育ios版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous (VSports)

