REVEAL-1, a phase 2 dose regimen optimization study of vosaroxin in older poor-risk patients with previously untreated acute myeloid leukaemia
- PMID: 25403830
- PMCID: "VSports注册入口" PMC4354261
- DOI: 10.1111/bjh.13214
REVEAL-1, a phase 2 dose regimen optimization study of vosaroxin in older poor-risk patients with previously untreated acute myeloid leukaemia
Abstract
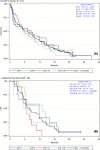
This phase 2 study (N = 116) evaluated single-agent vosaroxin, a first-in-class anticancer quinolone derivative, in patients ≥60 years of age with previously untreated unfavourable prognosis acute myeloid leukaemia. Dose regimen optimization was explored in sequential cohorts (A: 72 mg/m(2) d 1, 8, 15; B: 72 mg/m(2) d 1, 8; C: 72 mg/m(2) or 90 mg/m(2) d 1, 4). The primary endpoint was combined complete remission rate (complete remission [CR] plus CR with incomplete platelet recovery [CRp]). Common (>20%) grade ≥3 adverse events were thrombocytopenia, febrile neutropenia, anaemia, neutropenia, sepsis, pneumonia, stomatitis and hypokalaemia. Overall CR and CR/CRp rates were 29% and 32%; median overall survival (OS) was 7·0 months; 1-year OS was 34%. Schedule C (72 mg/m(2) ) had the most favourable safety and efficacy profile, with faster haematological recovery (median 27 d) and lowest incidence of aggregate sepsis (24%) and 30-d (7%) and 60-d (17%) all-cause mortality; at this dose and schedule, CR and CR/CRp rates were 31% and 35%, median OS was 7·7 months and 1-year OS was 38%. Overall, vosaroxin resulted in low early mortality and an encouraging response rate; vosaroxin 72 mg/m(2) d 1, 4 is recommended for further study in this population. Registered at www. clinicaltrials. gov: #NCT00607997 VSports手机版. .
Keywords: acute myeloid leukaemia; elderly; newly diagnosed; topoisomerase-II inhibitor; vosaroxin. V体育安卓版.
© 2014 The Authors. British Journal of Haematology published by John Wiley & Sons Ltd V体育ios版. .
Figures
References
-
- Blum W, Garzon R, Klisovic RB, Schwind S, Walker A, Geyer S, Liu S, Havelange V, Becker H, Schaaf L, Mickle J, Devine H, Kefauver C, Devine SM, Chan KK, Heerema NA, Bloomfield CD, Grever MR, Byrd JC, Villalona-Calero M, Croce CM. Marcucci G. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proceedings of the National Academy of Sciences USA. 2010;107:7473–7478. - VSports app下载 - PMC - PubMed
-
- Burnett A, Wetzler M. Lowenberg B. Therapeutic advances in acute myeloid leukemia. Journal of Clinical Oncology. 2011;29:487–494. - PubMed
-
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister TA, Lo-Coco F, Willemze R, Biondi A, Hiddemann W, Larson RA, Lowenberg B, Sanz MA, Head DR, Ohno R, Bloomfield CD International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Journal of Clinical Oncology. 2003;21:4642–4649. - PubMed
-
- Evanchik MJ, Allen D, Yoburn JC, Silverman JA. Hoch U. Metabolism of (+)-1,4-dihydro-7-(trans-3-methoxy-4-methylamino-1-pyrrolidinyl)-4-oxo-1-(2-thiaz olyl)-1,8-naphthyridine-3-carboxylic acid (voreloxin; formerly SNS-595), a novel replication-dependent DNA-damaging agent. Drug Metabolism and Disposition. 2009;37:594–601. - PubMed
Publication types
- Actions (VSports)
- VSports在线直播 - Actions
MeSH terms
- VSports app下载 - Actions
- Actions (VSports)
- "V体育官网入口" Actions
- Actions (V体育安卓版)
- Actions (VSports最新版本)
- Actions (VSports app下载)
- "VSports" Actions
- Actions (VSports注册入口)
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- Actions (V体育平台登录)
"VSports app下载" Substances
- V体育安卓版 - Actions
Associated data
- "VSports手机版" Actions
Grants and funding
LinkOut - more resources
"VSports在线直播" Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

