"V体育官网入口" Identification of factor H-like protein 1 as the predominant complement regulator in Bruch's membrane: implications for age-related macular degeneration
- PMID: 25305316
- PMCID: PMC4225158
- DOI: 10.4049/jimmunol.1401613 (V体育官网入口)
"VSports" Identification of factor H-like protein 1 as the predominant complement regulator in Bruch's membrane: implications for age-related macular degeneration
Abstract
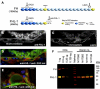
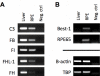
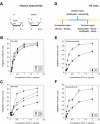
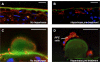
The tight regulation of innate immunity on extracellular matrix (ECM) is a vital part of immune homeostasis throughout the human body, and disruption to this regulation in the eye is thought to contribute directly to the progression of age-related macular degeneration (AMD) VSports手机版. The plasma complement regulator factor H (FH) is thought to be the main regulator that protects ECM against damaging complement activation. However, in the present study we demonstrate that a truncated form of FH, called FH-like protein 1 (FHL-1), is the main regulatory protein in the layer of ECM under human retina, called Bruch's membrane. Bruch's membrane is a major site of AMD disease pathogenesis and where drusen, the hallmark lesions of AMD, form. We show that FHL-1 can passively diffuse through Bruch's membrane, whereas the full sized, glycosylated, FH cannot. FHL-1 is largely bound to Bruch's membrane through interactions with heparan sulfate, and we show that the common Y402H polymorphism in the CFH gene, associated with an increased risk of AMD, reduces the binding of FHL-1 to this heparan sulfate. We also show that FHL-1 is retained in drusen whereas FH coats the periphery of the lesions, perhaps inhibiting their clearance. Our results identify a novel mechanism of complement regulation in the human eye, which highlights potential new avenues for therapeutic strategies. .
Copyright © 2014 by The American Association of Immunologists, Inc V体育安卓版. .
Figures

References
-
- Friedman DS, O’Colmain BJ, Muñoz B, Tomany SC, McCarty C, de Jong PTVM, Nemesure B, Mitchell P, Kempen J, E. D. P. R. Group Prevalence of age-related macular degeneration in the United States. Arch. Ophthalmol. 2004;122:564–572. - PubMed
-
- Anderson DH, Radeke MJ, Gallo NB, Chapin EA, Johnson PT, Curletti CR, Hancox LS, Hu J, Ebright JN, Malek G, Hauser MA, Rickman CB, Bok D, Hageman GS, Johnson LV. The pivotal role of the complement system in aging and age-related macular degeneration: Hypothesis re-visited. Prog. Retin. Eye Res. 2009;29:95–112. - PMC - PubMed
-
- Clark SJ, Bishop PN, Day AJ. Complement factor H and age-related macular degeneration: the role of glycosaminoglycan recognition in disease pathology. Biochem. Soc. Trans. 2010;38:1342–1348. - PubMed
-
- Whitcup SM, Sodhi A, Atkinson JP, Holers VM, Sinha D, Rohrer B, Dick AD. The Role of the Immune Response in Age-Related Macular Degeneration. Int. J. Inflam. 20132013:1–10. - "V体育平台登录" PMC - PubMed
Publication types
- "V体育2025版" Actions
"V体育安卓版" MeSH terms
- VSports注册入口 - Actions
- V体育官网 - Actions
- "V体育平台登录" Actions
- Actions (VSports手机版)
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
- VSports手机版 - Actions
- "VSports" Actions
- "VSports注册入口" Actions
- VSports注册入口 - Actions
- Actions (VSports)
- "V体育ios版" Actions
"V体育安卓版" Substances
- V体育官网入口 - Actions
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
"V体育平台登录" Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
VSports手机版 - Miscellaneous

