The cystic fibrosis transmembrane conductance regulator is an extracellular chloride sensor
- PMID: 25277268
- PMCID: PMC4502298
- DOI: 10.1007/s00424-014-1618-8
The cystic fibrosis transmembrane conductance regulator is an extracellular chloride sensor (VSports手机版)
"V体育平台登录" Abstract
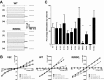
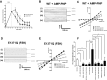
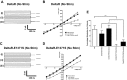
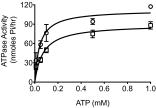
The cystic fibrosis transmembrane conductance regulator (CFTR) is a Cl(-) channel that governs the quantity and composition of epithelial secretions. CFTR function is normally tightly controlled as dysregulation can lead to life-threatening diseases such as secretory diarrhoea and cystic fibrosis VSports手机版. CFTR activity is regulated by phosphorylation of its cytosolic regulatory (R) domain, and ATP binding and hydrolysis at two nucleotide-binding domains (NBDs). Here, we report that CFTR activity is also controlled by extracellular Cl(-) concentration ([Cl(-)]o). Patch clamp current recordings show that a rise in [Cl(-)]o stimulates CFTR channel activity, an effect conferred by a single arginine residue, R899, in extracellular loop 4 of the protein. Using NBD mutants and ATP dose response studies in WT channels, we determined that [Cl(-)]o sensing was linked to changes in ATP binding energy at NBD1, which likely impacts NBD dimer stability. Biochemical measurements showed that increasing [Cl(-)]o decreased the intrinsic ATPase activity of CFTR mainly through a reduction in maximal ATP turnover. Our studies indicate that sensing [Cl(-)]o is a novel mechanism for regulating CFTR activity and suggest that the luminal ionic environment is an important physiological arbiter of CFTR function, which has significant implications for salt and fluid homeostasis in epithelial tissues. .
Figures


References
-
- Al-Nakkash L, Hu S, Li M, Hwang TC. A common mechanism for cystic fibrosis transmembrane conductance regulator protein activation by genistein and benzimidazolone analogs. J Pharmacol Exp Ther. 2001;296(2):464–472. - PubMed
-
- Aleksandrov L, Aleksandrov AA, Chang XB, Riordan JR. The first nucleotide binding domain of cystic fibrosis transmembrane conductance regulator is a site of stable nucleotide interaction, whereas the second is a site of rapid turnover. J Biol Chem. 2002;277(18):15419–15425. doi: 10.1074/jbc.M111713200. - VSports - DOI - PubMed
-
- Argent BE, Gray MA, Steward MC, Case RM (2012) In: L Johnson (ed) Physiology of the Gastrointestinal Tract. 5th edn. Elsevier, San Diego, pp 1399–1423
Publication types
- VSports最新版本 - Actions
V体育ios版 - MeSH terms
- "V体育2025版" Actions
- Actions (VSports最新版本)
- "VSports在线直播" Actions
- "VSports app下载" Actions
- VSports最新版本 - Actions
- Actions (VSports手机版)
- Actions (VSports注册入口)
- "VSports app下载" Actions
- Actions (VSports手机版)
- "V体育平台登录" Actions
Substances
- Actions (V体育平台登录)
- Actions (VSports)
- "V体育安卓版" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

