Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice
- PMID: 25251280
- PMCID: V体育安卓版 - PMC4340725
- DOI: VSports注册入口 - 10.1002/hep.27489
Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice
Abstract
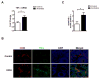
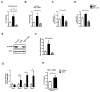
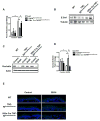
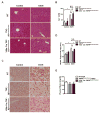
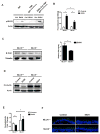
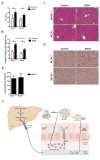
Intestinal barrier dysfunction is an important contributor to alcoholic liver disease (ALD). Translocated microbial products trigger an inflammatory response in the liver and contribute to steatohepatitis. Our aim was to investigate mechanisms of barrier disruption after chronic alcohol feeding VSports手机版. A Lieber-DeCarli model was used to induce intestinal dysbiosis, increased intestinal permeability, and liver disease in mice. Alcohol feeding for 8 weeks induced intestinal inflammation in the jejunum, which is characterized by an increased number of tumor necrosis factor alpha (TNF-α)-producing monocytes and macrophages. These findings were confirmed in duodenal biopsies from patients with chronic alcohol abuse. Intestinal decontamination with nonabsorbable antibiotics restored eubiosis, decreased intestinal inflammation and permeability, and reduced ALD in mice. TNF-receptor I (TNFRI) mutant mice were protected from intestinal barrier dysfunction and ALD. To investigate whether TNFRI on intestinal epithelial cells mediates intestinal barrier dysfunction and ALD, we used TNFRI mutant mice carrying a conditional gain-of-function allele for this receptor. Reactivation of TNFRI on intestinal epithelial cells resulted in increased intestinal permeability and liver disease that is similar to wild-type mice after alcohol feeding, suggesting that enteric TNFRI promotes intestinal barrier dysfunction. Myosin light-chain kinase (MLCK) is a downstream target of TNF-α and was phosphorylated in intestinal epithelial cells after alcohol administration. Using MLCK-deficient mice, we further demonstrate a partial contribution of MLCK to intestinal barrier dysfunction and liver disease after chronic alcohol feeding. .
Conclusion: Dysbiosis-induced intestinal inflammation and TNFRI signaling in intestinal epithelial cells mediate a disruption of the intestinal barrier. Therefore, intestinal TNFRI is a crucial mediator of ALD V体育安卓版. .
© 2014 by the American Association for the Study of Liver Diseases V体育ios版. .
"V体育ios版" Conflict of interest statement
None of the authors has a financial, personal or professional conflict of interest to disclose.
Figures

V体育官网 - Comment in
-
Tumor necrosis factor alpha-induced receptor 1 signaling in alcoholic liver disease: A gut reaction?Hepatology. 2015 Mar;61(3):754-6. doi: 10.1002/hep.27640. Epub 2015 Jan 28. Hepatology. 2015. PMID: 25482079 Free PMC article. No abstract available.
References
-
- Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–82. - PubMed
-
- Mathurin P, Deng QG, Keshavarzian A, Choudhary S, Holmes EW, Tsukamoto H. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–17. - PubMed
Publication types
- VSports app下载 - Actions
MeSH terms (V体育2025版)
- V体育官网 - Actions
- "V体育2025版" Actions
- VSports手机版 - Actions
- "VSports手机版" Actions
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- VSports注册入口 - Actions
Substances
- "V体育2025版" Actions
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
"VSports在线直播" Full Text Sources
Other Literature Sources (V体育官网)
