Deciphering the role of DC subsets in MCMV infection to better understand immune protection against viral infections
- PMID: 25120535
- PMCID: PMC4114203
- DOI: 10.3389/fmicb.2014.00378
Deciphering the role of DC subsets in MCMV infection to better understand immune protection against viral infections (V体育ios版)
Abstract
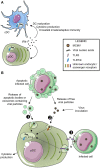
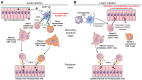
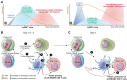
Infection of mice with murine cytomegalovirus (MCMV) recapitulates many physiopathological characteristics of human CMV infection and enables studying the interactions between a virus and its natural host. Dendritic cells (DC) are mononuclear phagocytes linking innate and adaptive immunity which are both necessary for MCMV control. DC are critical for the induction of cellular immunity because they are uniquely efficient for the activation of naïve T cells during their first encounter with a pathogen VSports手机版. DC are equipped with a variety of innate immune recognition receptors (I2R2) allowing them to detect pathogens or infections and to engulf molecules, microorganisms or cellular debris. The combinatorial engagement of I2R2 during infections controls DC maturation and shapes their response in terms of cytokine production, activation of natural killer (NK) cells and functional polarization of T cells. Several DC subsets exist which express different arrays of I2R2 and are specialized in distinct functions. The study of MCMV infection helped deciphering the physiological roles of DC subsets and their molecular regulation. It allowed the identification and first in vivo studies of mouse plasmacytoid DC which produce high level of interferons-α/β early after infection. Despite its ability to infect DC and dampen their functions, MCMV induces very robust, efficient and long-lasting CD8 T cell responses. Their priming may rely on the unique ability of uninfected XCR1(+) DC to cross-present engulfed viral antigens and thus to counter MCMV interference with antigen presentation. A balance appears to have been reached during co-evolution, allowing controlled replication of the virus for horizontal spread without pathological consequences for the immunocompetent host. We will discuss the role of the interplay between the virus and DC in setting this balance, and how advancing this knowledge further could help develop better vaccines against other intracellular infectious agents. .
Keywords: NK cells; XCR1+ dendritic cells; cross-presentation; immune evasion; murine cytomegalovirus; plasmacytoid dendritic cells; type I interferons; vaccination V体育安卓版. .
Figures

"V体育官网入口" References
-
- Adam C., King S., Allgeier T., Braumuller H., Luking C., Mysliwietz J., et al. (2005). DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 106, 338–344 10.1182/blood-2004-09-3775 - DOI (V体育官网) - PubMed
-
- Andoniou C. E., Van Dommelen S. L., Voigt V., Andrews D. M., Brizard G., Asselin-Paturel C., et al. (2005). Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat. Immunol. 6, 1011–1019 10.1038/ni1244 - DOI - PubMed
-
- Andrews D. M., Scalzo A. A., Yokoyama W. M., Smyth M. J., Degli-Esposti M. A. (2003). Functional interactions between dendritic cells and NK cells during viral infection. Nat. Immunol. 4, 175–181 10.1038/ni880 - VSports在线直播 - DOI - PubMed
Publication types (VSports app下载)
- V体育ios版 - Actions
Grants and funding
"V体育ios版" LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

