"V体育安卓版" Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis
- PMID: 24992962
- PMCID: PMC4117780
- DOI: 10.1093/nar/gku561
Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis (V体育安卓版)
Retraction in
-
Retraction: Long non-coding RNA INXS is a critical mediator of BCL-XS induced apoptosis.Nucleic Acids Res. 2016 Nov 2;44(19):9518. doi: 10.1093/nar/gkw713. Epub 2016 Aug 9. Nucleic Acids Res. 2016. PMID: 27507889 Free PMC article. No abstract available.
Abstract
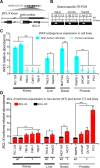
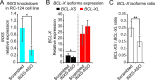
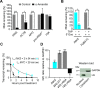
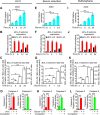
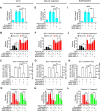
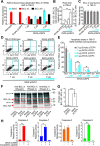
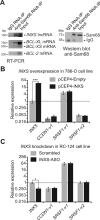
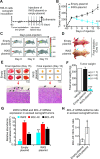
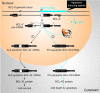
BCL-X mRNA alternative splicing generates pro-apoptotic BCL-XS or anti-apoptotic BCL-XL gene products and the mechanism that regulates splice shifting is incompletely understood. We identified and characterized a long non-coding RNA (lncRNA) named INXS, transcribed from the opposite genomic strand of BCL-X, that was 5- to 9-fold less abundant in tumor cell lines from kidney, liver, breast and prostate and in kidney tumor tissues compared with non-tumors. INXS is an unspliced 1903 nt-long RNA, is transcribed by RNA polymerase II, 5'-capped, nuclear enriched and binds Sam68 splicing-modulator. Three apoptosis-inducing agents increased INXS lncRNA endogenous expression in the 786-O kidney tumor cell line, increased BCL-XS/BCL-XL mRNA ratio and activated caspases 3, 7 and 9. These effects were abrogated in the presence of INXS knockdown. Similarly, ectopic INXS overexpression caused a shift in splicing toward BCL-XS and activation of caspases, thus leading to apoptosis. BCL-XS protein accumulation was detected upon INXS overexpression VSports手机版. In a mouse xenograft model, intra-tumor injections of an INXS-expressing plasmid caused a marked reduction in tumor weight, and an increase in BCL-XS isoform, as determined in the excised tumors. We revealed an endogenous lncRNA that induces apoptosis, suggesting that INXS is a possible target to be explored in cancer therapies. .
© The Author(s) 2014. Published by Oxford University Press on behalf of Nucleic Acids Research. V体育安卓版.
Figures
References
-
- Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. - PubMed
-
- Tait S.W., Green D.R. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat. Rev. Mol. Cell Biol. 2010;11:621–632. - PubMed (V体育官网入口)
-
- Boise L.H., Gonzalez-Garcia M., Postema C.E., Ding L., Lindsten T., Turka L.A., Mao X., Nunez G., Thompson C.B. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. - PubMed
Publication types
- VSports注册入口 - Actions
"V体育2025版" MeSH terms
- Actions (V体育平台登录)
- V体育平台登录 - Actions
- Actions (VSports app下载)
- Actions (V体育官网)
- VSports注册入口 - Actions
- Actions (V体育官网入口)
- Actions (VSports注册入口)
- "V体育2025版" Actions
- "VSports最新版本" Actions
- Actions (VSports注册入口)
- Actions (V体育平台登录)
- Actions (VSports)
- Actions (V体育官网)
"VSports手机版" Substances
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
- "V体育安卓版" Actions
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
"VSports app下载" Research Materials

