Endocytosis and exocytosis of nanoparticles in mammalian cells
- PMID: 24872703
- PMCID: PMC4024976
- DOI: 10.2147/IJN.S26592
Endocytosis and exocytosis of nanoparticles in mammalian cells
"V体育2025版" Abstract
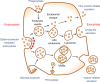
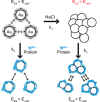
Engineered nanoparticles that can be injected into the human body hold tremendous potential to detect and treat complex diseases. Understanding of the endocytosis and exocytosis mechanisms of nanoparticles is essential for safe and efficient therapeutic application. In particular, exocytosis is of significance in the removal of nanoparticles with drugs and contrast agents from the body, while endocytosis is of great importance for the targeting of nanoparticles in disease sites VSports手机版. Here, we review the recent research on the endocytosis and exocytosis of functionalized nanoparticles based on various sizes, shapes, and surface chemistries. We believe that this review contributes to the design of safe nanoparticles that can efficiently enter and leave human cells and tissues. .
Keywords: cancer cell; drug delivery; endocytosis; exocytosis; macrophage; nanoparticle; toxicity V体育安卓版. .
"V体育安卓版" Figures



References
-
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171. - PubMed
-
- Park JH, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mat. 2009;8(4):331–336. - "V体育2025版" PMC - PubMed
Publication types (V体育ios版)
- Actions (VSports)
- VSports注册入口 - Actions
MeSH terms
- Actions (V体育官网入口)
- Actions (VSports在线直播)
- Actions (VSports app下载)
- VSports注册入口 - Actions
- Actions (VSports app下载)
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育官网)

