Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice
- PMID: 24857755
- PMCID: PMC4105345
- DOI: 10.1016/j.jim.2014.05.009
Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice
Abstract
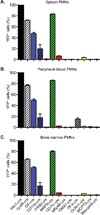
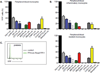
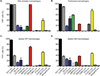
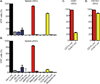
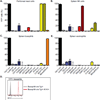
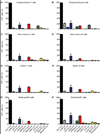
Since the first example of conditional gene targeting in mice in 1994, the use of Cre recombinase and loxP flanked sequences has become an invaluable technique to generate tissue and temporal specific gene knockouts. The number of mouse strains expressing floxed-gene sequences, and tissue-specific or temporal-specific Cre-recombinase that have been reported in the literature has grown exponentially. However, increased use of this technology has highlighted several problems that can impact the interpretation of any phenotype observed in these mouse models VSports手机版. In particular, accurate knowledge of the specificity of Cre expression in each strain is critical in order to make conclusions about the role of specific cell types in the phenotypes observed. Cre-mediated deletion specificity and efficiency have been described in many different ways in the literature, making direct comparisons between these Cre strains impossible. Here we report crossing thirteen different myeloid-Cre mouse strains to ROSA-EYFP reporter mice and assaying YFP expression in a variety of naïve unstimulated hematopoietic cells, in parallel. By focusing on myeloid subsets, we directly compare the relative efficiency and specificity of myeloid deletion in these strains under steady-state conditions. .
Keywords: Cre-recombinase; Dendritic cell; Floxed; Macrophage; Neutrophil V体育安卓版. .
Copyright © 2014 Elsevier B V体育ios版. V. All rights reserved. .
"V体育2025版" Figures

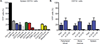

References
-
- Brockschnieder D, Pechmann Y, Sonnenberg-Riethmacher E, Riethmacher D. An improved mouse line for Cre-induced cell ablation due to diphtheria toxin A, expressed from the Rosa26 locus. Genesis. 2006;44:322–327. - VSports手机版 - PubMed
-
- Caton ML, Smith-Raska MR, Reizis B. Notch-RBP-J signaling controls the homeostasis of CD8− dendritic cells in the spleen. J Exp Med. 2007;204:1653–1664. - PMC (VSports) - PubMed
-
- Chisaka H, Morita E, Murata K, Ishii N, Yaegashi N, Okamura K, Sugamura K. A transgenic mouse model for non-immune hydrops fetalis induced by the NS1 gene of human parvovirus B19. The Journal of general virology. 2002;83:273–281. - "VSports最新版本" PubMed
Publication types (VSports注册入口)
VSports在线直播 - MeSH terms
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- "VSports在线直播" Actions
- "V体育平台登录" Actions
- VSports手机版 - Actions
- Actions (VSports在线直播)
V体育平台登录 - Substances
- "V体育2025版" Actions
- VSports - Actions
- "V体育平台登录" Actions
- Actions (VSports手机版)
Grants and funding
LinkOut - more resources (VSports app下载)
"V体育官网" Full Text Sources
"VSports在线直播" Other Literature Sources
Molecular Biology Databases

