Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice
- PMID: 24853068
- PMCID: PMC4121443
- DOI: 10.1007/s13311-013-0254-x (V体育2025版)
Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice
Abstract
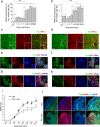
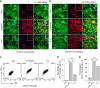
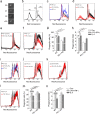
The inflammatory response following spinal cord injury (SCI) involves the activation of resident microglia and the infiltration of macrophages VSports手机版. Macrophages and microglia can be polarized into the classically activated proinflammatory M1 phenotype or the alternatively activated anti-inflammatory M2 phenotype. Programmed cell death 1 (PD-1) is a critical immune inhibitory receptor involved in innate and adaptive immune responses. However, whether PD-1 is involved in the modulation of macrophage/microglial polarization is unknown. In this study, the mRNA levels of pd1 gradually increased after SCI, and PD-1 protein was found in macrophages/microglia in injured spinal cord sections. PD-1 knockout (KO) mice showed poor locomotor recovery after spinal cord crushing compared with wild-type mice. M1-type macrophages/microglia accumulated in greater numbers in the injured spinal cord of PD-1-KO mice. Under polarized stimulation, induced expression of PD-1 occurred in cultured macrophages and microglia. PD-1 suppressed M1 polarization by reducing the phosphorylation of signal transducer and activator of transcription 1 (STAT1) and promoted M2 polarization by increasing STAT6 phosphorylation. In PD-1-KO mice, the M1 response was enhanced via the activation of STAT1 and nuclear factor-kappa B. Furthermore, PD-1 played various roles in phagocytosis in macrophages and microglia. Therefore, our results suggest that PD-1 signaling plays an important role in the regulation of macrophage/microglial polarization. Thus, deregulated PD-1 signaling may induce the polarization of macrophages/microglia toward the M1 phenotype. Overall, our results provide new insights into the modulatory mechanisms of macrophage/microglial polarization, thereby possibly facilitating the development of new therapies for SCI via the regulation of macrophage/microglial polarization through PD-1 signaling. .
Figures




References
-
- Chan CC. Inflammation: beneficial or detrimental after spinal cord injury? Recent Pat CNS Drug Discov. 2008;3:189–199. doi: 10.2174/157488908786242434. - DOI (VSports app下载) - PubMed
Publication types
MeSH terms
- V体育安卓版 - Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- Actions (VSports手机版)
- VSports app下载 - Actions
- "V体育ios版" Actions
- "V体育安卓版" Actions
Substances
- Actions (VSports手机版)
- Actions (V体育平台登录)
- Actions (V体育平台登录)
- "V体育2025版" Actions
V体育平台登录 - LinkOut - more resources
Full Text Sources
Other Literature Sources (VSports app下载)
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

