Bevacizumab plus ipilimumab in patients with metastatic melanoma
- PMID: 24838938
- PMCID: PMC4306338
- DOI: "V体育2025版" 10.1158/2326-6066.CIR-14-0053
Bevacizumab plus ipilimumab in patients with metastatic melanoma
Erratum in
- Cancer Immunol Res. 2014 Sep;2(9):923
"V体育ios版" Abstract
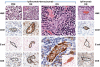
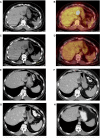
Ipilimumab improves survival in advanced melanoma and can induce immune-mediated tumor vasculopathy. Besides promoting angiogenesis, vascular endothelial growth factor (VEGF) suppresses dendritic cell maturation and modulates lymphocyte endothelial trafficking. This study investigated the combination of CTLA4 blockade with ipilimumab and VEGF inhibition with bevacizumab. Patients with metastatic melanoma were treated in four dosing cohorts of ipilimumab (3 or 10 mg/kg) with four doses at 3-week intervals and then every 12 weeks, and bevacizumab (7. 5 or 15 mg/kg) every 3 weeks. Forty-six patients were treated. Inflammatory events included giant cell arteritis (n = 1), hepatitis (n = 2), and uveitis (n = 2) VSports手机版. On-treatment tumor biopsies revealed activated vessel endothelium with extensive CD8(+) and macrophage cell infiltration. Peripheral blood analyses demonstrated increases in CCR7(+/-)/CD45RO(+) cells and anti-galectin antibodies. Best overall response included 8 partial responses, 22 instances of stable disease, and a disease-control rate of 67. 4%. Median survival was 25. 1 months. Bevacizumab influences changes in tumor vasculature and immune responses with ipilimumab administration. The combination of bevacizumab and ipilimumab can be safely administered and reveals VEGF-A blockade influences on inflammation, lymphocyte trafficking, and immune regulation. These findings provide a basis for further investigating the dual roles of angiogenic factors in blood vessel formation and immune regulation, as well as future combinations of antiangiogenesis agents and immune checkpoint blockade. .
©2014 American Association for Cancer Research. V体育安卓版.
Figures




References
-
- Robert C, Thomas L, Bondarenko I, O'Day S, Weber JW, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–26. - PubMed
-
- Ohm JE, Carbone DP. VEGF as a mediator of tumor-associated immunodeficiency. Immunol Res. 2001;23:263–72. - PubMed
-
- Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32. - PubMed
-
- Kandalaft LE, Motz GT, Busch J, Coukos G. Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol. 2011;344:129–48. - PubMed
V体育安卓版 - Publication types
MeSH terms
- V体育安卓版 - Actions
- VSports最新版本 - Actions
- Actions (V体育官网入口)
- "VSports app下载" Actions
- Actions (VSports在线直播)
- V体育ios版 - Actions
- V体育安卓版 - Actions
- VSports app下载 - Actions
- Actions (V体育官网)
- "V体育ios版" Actions
- V体育2025版 - Actions
- "V体育安卓版" Actions
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- Actions (VSports最新版本)
- V体育平台登录 - Actions
- V体育安卓版 - Actions
"VSports最新版本" Substances
- Actions (VSports注册入口)
VSports app下载 - Grants and funding
"VSports" LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

