Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature
- PMID: 24787605
- PMCID: PMC4224049
- DOI: 10.1089/ars.2013.5828
Reactive oxygen species deficiency induces autoimmunity with type 1 interferon signature
Abstract
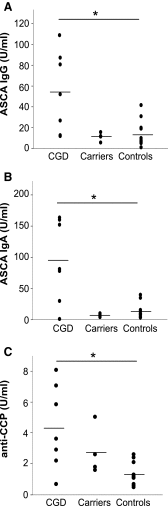
Aims: Chronic granulomatous disease (CGD) is a primary immunodeficiency caused by mutations in the phagocyte reactive oxygen species (ROS)-producing NOX2 enzyme complex and characterized by recurrent infections associated with hyperinflammatory and autoimmune manifestations. A translational, comparative analysis of CGD patients and the corresponding ROS-deficient Ncf1(m1J) mutated mouse model was performed to reveal the molecular pathways operating in NOX2 complex deficient inflammation. VSports手机版.
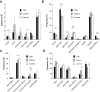
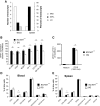
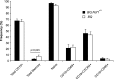
Results: A prominent type I interferon (IFN) response signature that was accompanied by elevated autoantibody levels was identified in both mice and humans lacking functional NOX2 complex. To further underline the systemic lupus erythematosus (SLE)-related autoimmune process, we show that naïve Ncf1(m1J) mutated mice, similar to SLE patients, suffer from inflammatory kidney disease with IgG and C3 deposits in the glomeruli V体育安卓版. Expression analysis of germ-free Ncf1(m1J) mutated mice reproduced the type I IFN signature, enabling us to conclude that the upregulated signaling pathway is of endogenous origin. .
Innovation: Our findings link the previously unexplained connection between ROS deficiency and increased susceptibility to autoimmunity by the discovery that activation of IFN signaling is a major pathway downstream of a deficient NOX2 complex in both mice and humans V体育ios版. .
Conclusion: We conclude that the lack of phagocyte-derived oxidative burst is associated with spontaneous autoimmunity and linked with type I IFN signature in both mice and humans. VSports最新版本.
Figures



References (VSports)
-
- Al-Herz W, Bousfiha A, Casanova JL, Chapel H, Conley ME, Cunningham-Rundles C, Etzioni A, Fischer A, Franco JL, Geha RS, Hammarstrom L, Nonoyama S, Notarangelo LD, Ochs HD, Puck JM, Roifman CM, Seger R, and Tang ML. Primary immunodeficiency diseases: an update on the classification from the international union of immunological societies expert committee for primary immunodeficiency. Front Immunol 2: 54, 2011 - VSports手机版 - PMC - PubMed
-
- Arbuckle MR, McClain MT, Rubertone MV, Scofield RH, Dennis GJ, James JA, and Harley JB. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 349: 1526–1533, 2003 - PubMed
-
- Badolato R, Notarangelo LD, Plebani A, and Roos D. Development of systemic lupus erythematosus in a young child affected with chronic granulomatous disease following withdrawal of treatment with interferon-gamma. Rheumatology (Oxford) 42: 804–805, 2003 - PubMed
-
- Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, and Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci U S A 100: 2610–2615, 2003 - PMC - PubMed
Publication types
MeSH terms
- "VSports app下载" Actions
- V体育安卓版 - Actions
- "V体育2025版" Actions
- Actions (V体育安卓版)
- Actions (V体育2025版)
- V体育官网 - Actions
- "VSports app下载" Actions
- VSports手机版 - Actions
- "V体育安卓版" Actions
Substances
- V体育安卓版 - Actions
- VSports手机版 - Actions
- Actions (V体育官网)
- V体育官网 - Actions
- Actions (V体育安卓版)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

