Observation of a Cu(II)(2) (μ-1,2-peroxo)/Cu(III)(2) (μ-oxo)(2) equilibrium and its implications for copper-dioxygen reactivity
- PMID: 24700427
- PMCID: PMC4041702
- DOI: 10.1002/anie.201402166
Observation of a Cu(II)(2) (μ-1,2-peroxo)/Cu(III)(2) (μ-oxo)(2) equilibrium and its implications for copper-dioxygen reactivity
Abstract
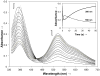
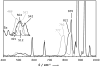
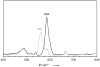
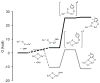
Synthesis of small-molecule Cu2 O2 adducts has provided insight into the related biological systems and their reactivity patterns including the interconversion of the Cu(II) 2 (μ-η(2) :η(2) -peroxo) and Cu(III) 2 (μ-oxo)2 isomers VSports手机版. In this study, absorption spectroscopy, kinetics, and resonance Raman data show that the oxygenated product of [(BQPA)Cu(I) ](+) initially yields an "end-on peroxo" species, that subsequently converts to the thermodynamically more stable "bis-μ-oxo" isomer (Keq =3. 2 at -90 °C). Calibration of density functional theory calculations to these experimental data suggest that the electrophilic reactivity previously ascribed to end-on peroxo species is in fact a result of an accessible bis-μ-oxo isomer, an electrophilic Cu2 O2 isomer in contrast to the nucleophilic reactivity of binuclear Cu(II) end-on peroxo species. This study is the first report of the interconversion of an end-on peroxo to bis-μ-oxo species in transition metal-dioxygen chemistry. .
Keywords: Raman spectroscopy; copper; dioxygen ligands; structure-function relationship; tyrosinase V体育安卓版. .
© 2014 WILEY-VCH Verlag GmbH & Co V体育ios版. KGaA, Weinheim. .
Figures (V体育2025版)
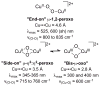
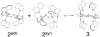

References
-
- Solomon EI, et al. Chem Rev. 2014 doi: 10.1021/cr400327t. - DOI - PubMed
- Ginsbach JW, Kieber-Emmons MT, Nomoto R, Noguchi A, Ohnishi Y, Solomon EI. Proceedings of the National Academy of Sciences. 2012;109:10793–10797. - "VSports注册入口" PMC - PubMed
-
- Kitajima N, Moro-oka Y. Chem Rev. 1994;94:737–757.
- Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. - PubMed
- Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. - VSports注册入口 - PubMed
- Hatcher LQ, Karlin KD. J Biol Inorg Chem. 2004;9:669–683. - V体育ios版 - PubMed
-
- Nasir MS, Cohen BI, Karlin KD. J Am Chem Soc. 1992;114:2482–2494.
- Pidcock E, Obias HV, Zhang CX, Karlin KD, Solomon EI. J Am Chem Soc. 1998;120:7841–7847.
- Holland PL, Rodgers KR, Tolman WB. Angew Chem Int Ed Engl. 1999;38:1139–1142. - PubMed
- Mirica LM, Vance MA, Rudd DJ, Hedman B, Hodgson KO, Solomon EI, Stack TDP. Science. 2005;308:1890–1892. - PubMed
- Company A, Palavicini S, Garcia-Bosch I, Mas-Balleste R, Que L, Rybak-Akimova EV, Casella L, Ribas X, Costas M. Chem-Eur J. 2008;14:3535–3538. - PubMed
- Qayyum MF, Sarangi R, Fujisawa K, Stack TDP, Karlin KD, Hodgson KO, Hedman B, Solomon EI. J Am Chem Soc. 2013;135:17417–17431. - PMC - PubMed
-
- Paul PP, Tyeklár Z, Jacobson RR, Karlin KD. J Am Chem Soc. 1991;113:5322–5332.
Publication types (VSports app下载)
- Actions (VSports)
MeSH terms
- "VSports app下载" Actions
- "VSports最新版本" Actions
V体育2025版 - Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
"VSports在线直播" Miscellaneous

