Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice
- PMID: 24664548
- PMCID: PMC4474376
- DOI: 10.1002/art.38639
Fibrin accumulation secondary to loss of plasmin-mediated fibrinolysis drives inflammatory osteoporosis in mice
Abstract
Objective: Osteoporosis is a skeletal disorder characterized by low bone mass and increased bone fragility associated with aging, menopause, smoking, obesity, or diabetes. Persistent inflammation has been identified as an instigating factor in progressive bone loss. In addition to the role of fibrin in coagulation, inordinate fibrin deposition within a tissue matrix results in increased local inflammation. Given that fibrin accumulation is a hallmark of osteoporosis-related comorbidities, we undertook this study to test the hypothesis that persistent fibrin deposition causes inflammatory osteoporosis VSports手机版. .
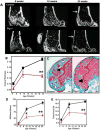
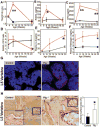
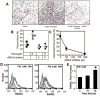
Methods: Multiple imaging modalities, bone integrity metrics, and histologic analyses were employed to evaluate skeletal derangements in relation to fibrin deposition, circulating fibrinogen levels, and systemic markers of inflammation in mice that were plasminogen deficient and in plasminogen-deficient mice that were concomitantly either fibrinogen deficient or carrying a mutant form of fibrinogen lacking the αM β2 binding motif V体育安卓版. .
Results: Mice generated with a genetic deficit in the key fibrinolytic protease, plasmin, uniformly developed severe osteoporosis. Furthermore, the development of osteoporosis was fibrin(ogen) dependent, and the derangements in the bone remodeling unit were mechanistically tied to fibrin(ogen)-mediated activation of osteoclasts via activation of the leukocyte integrin receptor αM β2 on monocytes and secondary stimulation of osteoblasts by RANKL. Notably, the genetic elimination of fibrin(ogen) or the expression of a mutant form of fibrinogen retaining clotting function but lacking the αM β2 binding motif prevented the degenerative skeletal phenotypes, resulting in normal local and systemic cytokine levels. V体育ios版.
Conclusion: Taken together, these data reveal for the first time that fibrin promotes inflammation-driven systemic osteoporosis, which suggests a novel association between hemostasis, inflammation, and bone biology VSports最新版本. .
Copyright © 2014 by the American College of Rheumatology V体育平台登录. .
Figures (VSports注册入口)


References
-
- Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–16. - PubMed
-
- Mundy GR. Osteoporosis and inflammation. Nutr Rev. 2007;65:S147–51. - PubMed
-
- Daniell HW. Osteoporosis and smoking. JAMA. 1972;221:509. - PubMed
-
- Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J Bone Miner Res. 2007;22:1317–28. - PubMed
Publication types
MeSH terms
- VSports - Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
VSports注册入口 - Molecular Biology Databases

