TNFR1/phox interaction and TNFR1 mitochondrial translocation Thwart silica-induced pulmonary fibrosis
- PMID: 24623132
- PMCID: PMC3977215
- DOI: 10.4049/jimmunol.1103516
TNFR1/phox interaction and TNFR1 mitochondrial translocation Thwart silica-induced pulmonary fibrosis
V体育ios版 - Abstract
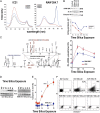
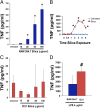
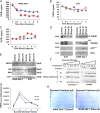
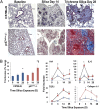
Macrophages play a fundamental role in innate immunity and the pathogenesis of silicosis. Phagocytosis of silica particles is associated with the generation of reactive oxygen species (ROS), secretion of cytokines, such as TNF, and cell death that contribute to silica-induced lung disease VSports手机版. In macrophages, ROS production is executed primarily by activation of the NADPH oxidase (Phox) and by generation of mitochondrial ROS (mtROS); however, the relative contribution is unclear, and the effects on macrophage function and fate are unknown. In this study, we used primary human and mouse macrophages (C57BL/6, BALB/c, and p47(phox-/-)) and macrophage cell lines (RAW 264. 7 and IC21) to investigate the contribution of Phox and mtROS to silica-induced lung injury. We demonstrate that reduced p47(phox) expression in IC21 macrophages is linked to enhanced mtROS generation, cardiolipin oxidation, and accumulation of cardiolipin hydrolysis products, culminating in cell death. mtROS production is also observed in p47(phox-/-) macrophages, and p47(phox-/-) mice exhibit increased inflammation and fibrosis in the lung following silica exposure. Silica induces interaction between TNFR1 and Phox in RAW 264. 7 macrophages. Moreover, TNFR1 expression in mitochondria decreased mtROS production and increased RAW 264. 7 macrophage survival to silica. These results identify TNFR1/Phox interaction as a key event in the pathogenesis of silicosis that prevents mtROS formation and reduces macrophage apoptosis. .
Figures

References
-
- Cassel S. L., Eisenbarth S. C., Iyer S. S., Sadler J. J., Colegio O. R., Tephly L. A., Carter A. B., Rothman P. B., Flavell R. A., Sutterwala F. S. 2008. The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 105: 9035–9040 - PMC (VSports注册入口) - PubMed
VSports在线直播 - Publication types
- Actions (VSports手机版)
MeSH terms
- V体育安卓版 - Actions
- "V体育2025版" Actions
- VSports注册入口 - Actions
- Actions (VSports最新版本)
- Actions (VSports手机版)
- Actions (V体育官网入口)
- VSports - Actions
- Actions (V体育安卓版)
- Actions (V体育安卓版)
- "V体育安卓版" Actions
- V体育2025版 - Actions
Substances
- "VSports最新版本" Actions
- Actions (V体育ios版)
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
V体育安卓版 - Other Literature Sources
Molecular Biology Databases

