"VSports" Role of transcription factor acetylation in diabetic kidney disease
- PMID: 24608443
- PMCID: PMC4066331
- DOI: "VSports注册入口" 10.2337/db13-1810
"V体育ios版" Role of transcription factor acetylation in diabetic kidney disease
Abstract
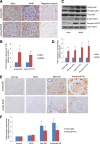
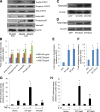
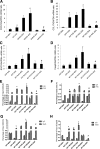
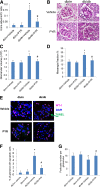
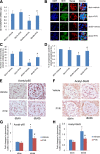
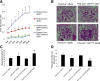
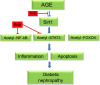
Nuclear factor (NF)-κB and signal transducer and activator of transcription 3 (STAT3) play a critical role in diabetic nephropathy (DN). Sirtuin-1 (SIRT1) regulates transcriptional activation of target genes through protein deacetylation. Here, we determined the roles of Sirt1 and the effect of NF-κB (p65) and STAT3 acetylation in DN. We found that acetylation of p65 and STAT3 was increased in both mouse and human diabetic kidneys. In human podocytes, advanced glycation end products (AGEs) induced p65 and STAT3 acetylation and overexpression of acetylation-incompetent mutants of p65 and STAT3 abrogated AGE-induced expression of NF-κB and STAT3 target genes VSports手机版. Inhibition of AGE formation in db/db mice by pyridoxamine treatment attenuated proteinuria and podocyte injury, restored SIRT1 expression, and reduced p65 and STAT3 acetylation. Diabetic db/db mice with conditional deletion of SIRT1 in podocytes developed more proteinuria, kidney injury, and acetylation of p65 and STAT3 compared with db/db mice without SIRT1 deletion. Treatment of db/db mice with a bromodomain and extraterminal (BET)-specific bromodomain inhibitor (MS417) which blocks acetylation-mediated association of p65 and STAT3 with BET proteins, attenuated proteinuria, and kidney injury. Our findings strongly support a critical role for p65 and STAT3 acetylation in DN. Targeting protein acetylation could be a potential new therapy for DN. .
© 2014 by the American Diabetes Association. V体育安卓版.
"VSports注册入口" Figures

References
-
- Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2010 annual data report. Am J Kidney Dis 2011;57:A8, e1–526 - PubMed (VSports注册入口)
-
- Bohlender JM, Franke S, Stein G, Wolf G. Advanced glycation end products and the kidney. Am J Physiol Renal Physiol 2005;289:F645–F659 - PubMed (V体育平台登录)
-
- Zheng F, Zeng YJ, Plati AR, et al. Combined AGE inhibition and ACEi decreases the progression of established diabetic nephropathy in B6 db/db mice. Kidney Int 2006;70:507–514 - PubMed
"V体育安卓版" MeSH terms
- "VSports手机版" Actions
- V体育2025版 - Actions
- "VSports在线直播" Actions
- V体育平台登录 - Actions
Substances
- Actions (V体育安卓版)
- V体育2025版 - Actions
- Actions (V体育平台登录)
- Actions (V体育官网入口)
- VSports app下载 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (VSports)
Miscellaneous

