Lag structure in resting-state fMRI
- PMID: 24598530
- PMCID: PMC4097876
- DOI: 10.1152/jn.00804.2013
Lag structure in resting-state fMRI
"V体育ios版" Abstract
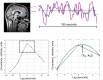
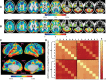
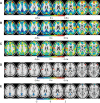
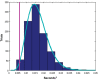
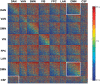
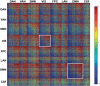
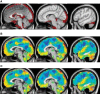
The discovery that spontaneous fluctuations in blood oxygen level-dependent (BOLD) signals contain information about the functional organization of the brain has caused a paradigm shift in neuroimaging. It is now well established that intrinsic brain activity is organized into spatially segregated resting-state networks (RSNs). Less is known regarding how spatially segregated networks are integrated by the propagation of intrinsic activity over time. To explore this question, we examined the latency structure of spontaneous fluctuations in the fMRI BOLD signal. Our data reveal that intrinsic activity propagates through and across networks on a timescale of ∼1 s. Variations in the latency structure of this activity resulting from sensory state manipulation (eyes open vs VSports手机版. closed), antecedent motor task (button press) performance, and time of day (morning vs. evening) suggest that BOLD signal lags reflect neuronal processes rather than hemodynamic delay. Our results emphasize the importance of the temporal structure of the brain's spontaneous activity. .
Keywords: dynamics; fMRI; functional connectivity; resting state V体育安卓版. .
Copyright © 2014 the American Physiological Society. V体育ios版.
Figures






References
-
- Allison M, Bodine E, DeAlba LM, Debnath J, DeLoss L, Garnett C, Grout J, Hogben L, Im B, Kim H, Nair R, Pryporova O, Savage K, Shader B, Wehe AW. Minimum rank of skew-symmetric matrices described by a graph. Linear Algebra Appl 432: 2457–2472, 2010
-
- Amemiya S, Kunimatsu A, Saito N, Ohtomo K. Cerebral hemodynamic impairment: assessment with resting-state functional MR imaging. Radiology 270: 548–555, 2014 - PubMed
-
- Axmacher N, Draguhn A, Elger CE, Fell J. Memory processes during sleep: beyond the standard consolidation theory. Cell Mol Life Sci 66: 2285–2297, 2009 - "VSports手机版" PMC - PubMed
MeSH terms
- V体育平台登录 - Actions
- Actions (VSports app下载)
- "V体育官网入口" Actions
- "V体育官网" Actions
- "V体育官网" Actions
- V体育ios版 - Actions
- V体育平台登录 - Actions
- V体育官网 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

