Z α1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease
- PMID: 24592811
- PMCID: PMC4098095
- DOI: 10.1164/rccm.201308-1458OC
Z α1-antitrypsin confers a proinflammatory phenotype that contributes to chronic obstructive pulmonary disease
V体育安卓版 - Abstract
Rationale: Severe α1-antitrypsin deficiency caused by the Z variant (Glu342Lys; ZZ-AT) is a well-known genetic cause for emphysema VSports手机版. Although severe lack of antiproteinase protection is the critical etiologic factor for ZZ-AT-associated chronic obstructive pulmonary disease (COPD), some reports have suggested enhanced lung inflammation as a factor in ZZ-AT homozygotes. .
Objectives: To provide molecular characterization of inflammation in ZZ-AT. V体育安卓版.
Methods: Inflammatory cell and cytokine profile (nuclear factor-κB, IL-6, tumor necrosis factor-α), intracellular polymerization of Z-AT, and endoplasmic reticulum (ER) stress markers (protein kinase RNA-like ER kinase, activator transcription factor 4) were assessed in transgenic mice and transfected cells in response to cigarette smoke, and in explanted lungs from ZZ and MM individuals with severe COPD V体育ios版. .
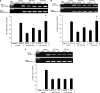
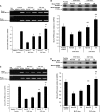
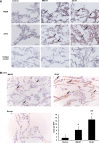
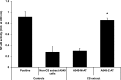
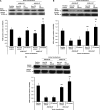
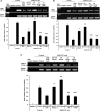
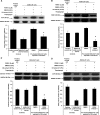
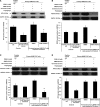
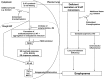
Measurements and main results: Compared with M-AT, transgenic Z-AT mice lungs exposed to cigarette smoke had higher levels of pulmonary cytokines, neutrophils, and macrophages and an exaggerated ER stress. Similarly, the ER overload response was greater in lungs from ZZ-AT homozygotes with COPD, and was particularly found in pulmonary epithelial cells. Cigarette smoke increased intracellular Z-AT polymers, ER overload response, and proinflammatory cytokine release in Z-AT-expressing pulmonary epithelial cells, which could be prevented with an inhibitor of polymerization, an antioxidant, and an inhibitor of protein kinase RNA-like ER kinase. VSports最新版本.
Conclusions: We show here that aggregation of intracellular mutant Z-AT invokes a specific deleterious cellular inflammatory phenotype in COPD. Oxidant-induced intracellular polymerization of Z-AT in epithelial cells causes ER stress, and promotes excess cytokine and cellular inflammation. This pathway is likely to contribute to the development of COPD in ZZ-AT homozygotes, and therefore merits further investigation V体育平台登录. .
Figures







V体育2025版 - Comment in
-
Oxidant-mediated aggregation of Z α1-antitrypsin in pulmonary epithelial cells amplifies lung inflammation.Am J Respir Crit Care Med. 2014 Apr 15;189(8):877-9. doi: 10.1164/rccm.201403-0402ED. Am J Respir Crit Care Med. 2014. PMID: 24735027 No abstract available.
References
-
- Stoller JK, Aboussouan LS. A review of α1-antitrypsin deficiency. Am J Respir Crit Care Med. 2012;185:246–259. - PubMed
-
- Eriksson S. Studies in α 1-antitrypsin deficiency. Acta Med Scand Suppl. 1965;432:1–85. - PubMed
-
- Blanco I, de Serres FJ, Fernandez-Bustillo E, Lara B, Miravitlles M. Estimated numbers and prevalence of PI*S and PI*Z alleles of alpha1-antitrypsin deficiency in European countries. Eur Respir J. 2006;27:77–84. - PubMed
-
- de Serres FJ, Blanco I, Fernández-Bustillo E. Genetic epidemiology of alpha-1 antitrypsin deficiency in North America and Australia/New Zealand: Australia, Canada, New Zealand and the United States of America. Clin Genet. 2003;64:382–397. - PubMed
-
- Lomas DA, Evans DL, Finch JT, Carrell RW. The mechanism of Z alpha 1-antitrypsin accumulation in the liver. Nature. 1992;357:605–607. - PubMed
Publication types
MeSH terms
- V体育ios版 - Actions
- "VSports注册入口" Actions
- "V体育官网入口" Actions
- "VSports手机版" Actions
- Actions (V体育官网)
- "VSports注册入口" Actions
- "V体育平台登录" Actions
- "VSports app下载" Actions
- V体育2025版 - Actions
- "VSports在线直播" Actions
- Actions (VSports注册入口)
- VSports手机版 - Actions
Substances
- Actions (V体育安卓版)
- "V体育2025版" Actions
- Actions (VSports)
- VSports在线直播 - Actions
Grants and funding
LinkOut - more resources
"V体育ios版" Full Text Sources
Other Literature Sources
Medical (VSports手机版)

