Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma
- PMID: 24586047
- PMCID: PMC3977239
- DOI: 10.1073/pnas.1319963111
VSports最新版本 - Multiple cells-of-origin of mutant K-Ras-induced mouse lung adenocarcinoma
Abstract
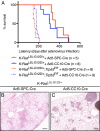
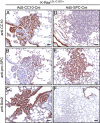
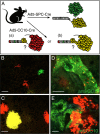
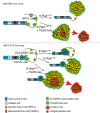
Much controversy surrounds the cell-of-origin of mutant K-Ras (K-RasG12D)-induced lung adenocarcinoma. To shed light on this issue, we have used technology that enables us to conditionally target K-RasG12D expression in Surfactant Protein C (SPC)(+) alveolar type 2 cells and in Clara cell antigen 10 (CC10)(+) Clara cells by use of cell-type-restricted recombinant Adeno-Cre viruses. Experiments were performed both in the presence and absence of the tumor suppressor gene p53, enabling us to assess what effect the cell-of-origin and the introduced genetic lesions have on the phenotypic characteristics of the resulting adenocarcinomas. We conclude that both SPC-expressing alveolar type 2 cells and CC10-expressing Clara cells have the ability to initiate malignant transformation following the introduction of these genetic alterations. The lungs of K-Ras(lox-Stop-lox-G12D/+) and K-Ras(lox-Stop-lox-G12D/+);tumor suppressor gene Trp53(F/F) mice infected with Adeno5-SPC-Cre and Adeno5-CC10-Cre viruses displayed differences in their tumor spectrum, indicating distinct cellular routes of tumor initiation. Moreover, using a multicolor Cre reporter line, we demonstrate that the resulting tumors arise from a clonal expansion of switched cells. Taken together, these results indicate that there are multiple cellular paths to K-RasG12D-induced adenocarcinoma and that the initiating cell influences the histopathological phenotype of the tumors that arise VSports手机版. .
Keywords: BASCs; NSCLC; bronchioalveolar stem cells; non small cell lung cancer. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Diverse cells at the origin of lung adenocarcinoma.Proc Natl Acad Sci U S A. 2014 Apr 1;111(13):4745-6. doi: 10.1073/pnas.1401955111. Epub 2014 Mar 20. Proc Natl Acad Sci U S A. 2014. PMID: 24707043 Free PMC article. No abstract available.
V体育官网入口 - References
-
- Mao C, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: A meta-analysis of 22 studies. Lung Cancer. 2010;69(3):272–278. - "VSports手机版" PubMed
-
- Kim CF, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. - PubMed
-
- Ventura JJ, et al. p38alpha MAP kinase is essential in lung stem and progenitor cell proliferation and differentiation. Nat Genet. 2007;39(6):750–758. - PubMed (VSports)
Publication types
- V体育官网 - Actions
MeSH terms
- Actions (V体育平台登录)
- "V体育官网" Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- Actions (V体育官网入口)
- "VSports最新版本" Actions
- Actions (V体育安卓版)
- VSports app下载 - Actions
- "VSports在线直播" Actions
- VSports最新版本 - Actions
- "V体育平台登录" Actions
"VSports最新版本" Substances
- "VSports" Actions
- "V体育2025版" Actions
- "VSports在线直播" Actions
- VSports在线直播 - Actions
- Actions (V体育2025版)
- "VSports手机版" Actions
VSports手机版 - LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
V体育官网 - Molecular Biology Databases
Research Materials
"VSports手机版" Miscellaneous

