Endogenous interleukin (IL)-17A promotes pristane-induced systemic autoimmunity and lupus nephritis induced by pristane
- PMID: 24528105
- PMCID: PMC4008978
- DOI: 10.1111/cei.12287
Endogenous interleukin (IL)-17A promotes pristane-induced systemic autoimmunity and lupus nephritis induced by pristane (VSports注册入口)
Abstract
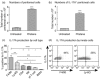
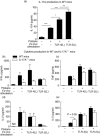
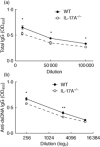
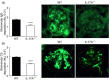
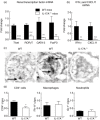
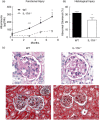
Interleukin (IL)-17A is increased both in serum and in kidney biopsies from patients with lupus nephritis, but direct evidence of pathogenicity is less well established. Administration of pristane to genetically intact mice results in the production of autoantibodies and proliferative glomerulonephritis, resembling human lupus nephritis. These studies sought to define the role of IL-17A in experimental lupus induced by pristane administration. Pristane was administered to wild-type (WT) and IL-17A(-/-) mice. Local and systemic immune responses were assessed after 6 days and 8 weeks, and autoimmunity, glomerular inflammation and renal injury were measured at 7 months. IL-17A production increased significantly 6 days after pristane injection, with innate immune cells, neutrophils (Ly6G(+)) and macrophages (F4/80(+)) being the predominant source of IL-17A. After 8 weeks, while systemic IL-17A was still readily detected in WT mice, the levels of proinflammatory cytokines, interferon (IFN)-γ and tumour necrosis factor (TNF) were diminished in the absence of endogenous IL-17A. Seven months after pristane treatment humoral autoimmunity was diminished in the absence of IL-17A, with decreased levels of immunoglobulin (Ig)G and anti-dsDNA antibodies. Renal inflammation and injury was less in the absence of IL-17A. Compared to WT mice, glomerular IgG, complement deposition, glomerular CD4(+) T cells and intrarenal expression of T helper type 1 (Th1)-associated proinflammatory mediators were decreased in IL-17A(-/-) mice VSports手机版. WT mice developed progressive proteinuria, but functional and histological renal injury was attenuated in the absence of IL-17A. Therefore, IL-17A is required for the full development of autoimmunity and lupus nephritis in experimental SLE, and early in the development of autoimmunity, innate immune cells produce IL-17A. .
Keywords: glomerulonephritis; interleukin 17A; lupus nephritis; pristane; systemic lupus erythematosus. V体育安卓版.
© 2014 British Society for Immunology.
Figures
References
-
- Ward MM, Pyun E, Studenski S. Mortality risks associated with specific clinical manifestations of systemic lupus erythematosus. Arch Intern Med. 1996;156:1337–1344. - PubMed
-
- Koffler D, Agnello V, Thoburn R, Kunkel HG. Systemic lupus erythematosus: prototype of immune complex nephritis in man. J Exp Med. 1971;134:169s–179. - PubMed
-
- Alexopoulos E, Seron D, Hartley RB, Cameron JS. Lupus nephritis: correlation of interstitial cells with glomerular function. Kidney Int. 1990;37:100–109. - PubMed
-
- Anders HJ, Schlondorff DO. Innate immune receptors and autophagy: implications for autoimmune kidney injury. Kidney Int. 2010;78:29–37. - PubMed
Publication types
MeSH terms
- "V体育ios版" Actions
- "VSports" Actions
- Actions (V体育官网)
- Actions (VSports)
- Actions (VSports在线直播)
- "VSports app下载" Actions
- "VSports" Actions
- VSports手机版 - Actions
- Actions (V体育ios版)
- "VSports在线直播" Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- V体育2025版 - Actions
- VSports在线直播 - Actions
- Actions (V体育安卓版)
- VSports最新版本 - Actions
Substances
- Actions (V体育安卓版)
- "V体育安卓版" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials (V体育平台登录)

