Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin
- PMID: 24526740
- PMCID: PMC4091580
- DOI: V体育2025版 - 10.1093/infdis/jiu091
Increased neutrophil extracellular trap-mediated Staphylococcus aureus clearance through inhibition of nuclease activity by clindamycin and immunoglobulin
Abstract
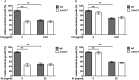
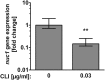
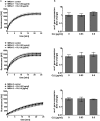
The Gram-positive human pathogen Staphylococcus aureus causes a variety of human diseases such as skin infections, pneumonia, and endocarditis. The micrococcal nuclease Nuc1 is one of the major S VSports手机版. aureus virulence factors and allows the bacterium to avoid neutrophil extracellular trap (NET)-mediated killing. We found that addition of the protein synthesis inhibitor clindamycin to S. aureus LAC cultures decreased nuc1 transcription and subsequently blunted nuclease activity in a molecular beacon-based fluorescence assay. We also observed reduced NET degradation through Nuc1 inhibition translating into increased NET-mediated clearance. Similarly, pooled human immunoglobulin specifically inhibited nuclease activity in a concentration-dependent manner. Inhibition of nuclease activity by clindamycin and immunoglobulin enhanced S. aureus clearance and should be considered in the treatment of S. aureus infections. .
Keywords: NET; Staphylococcus aureus; clindamycin; immunoglobulin; molecular beacon; nuclease. V体育安卓版.
© The Author 2014 V体育ios版. Published by Oxford University Press on behalf of the Infectious Diseases Society of America. .
Figures



References
-
- Lowy FD. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–32. - PubMed
-
- Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352:1445–53. - PubMed
-
- Francis JS, Doherty MC, Lopatin U, et al. Severe community-onset pneumonia in healthy adults caused by methicillin-resistant Staphylococcus aureus carrying the Panton-Valentine leukocidin genes. Clin Infect Dis. 2005;40:100–7. - PubMed (V体育官网入口)
-
- Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis. 2011;52:e18–55. - PubMed
Publication types
- "V体育ios版" Actions
MeSH terms
- "V体育官网入口" Actions
- Actions (V体育平台登录)
- Actions (V体育ios版)
- VSports - Actions
- VSports注册入口 - Actions
- "VSports最新版本" Actions
- "V体育安卓版" Actions
Substances
LinkOut - more resources
Full Text Sources
"VSports手机版" Other Literature Sources

