Suppression of tumor angiogenesis by targeting the protein neddylation pathway
- PMID: 24525735
- PMCID: V体育安卓版 - PMC3944239
- DOI: 10.1038/cddis.2014.21 (V体育平台登录)
V体育平台登录 - Suppression of tumor angiogenesis by targeting the protein neddylation pathway
Abstract
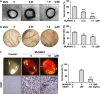
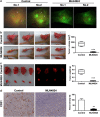
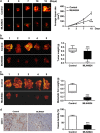
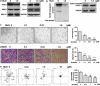
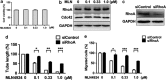
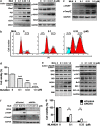
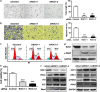
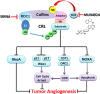
Inhibition of protein neddylation, particularly cullin neddylation, has emerged as a promising anticancer strategy, as evidenced by the antitumor activity in preclinical studies of the Nedd8-activating enzyme (NAE) inhibitor MLN4924. This small molecule can block the protein neddylation pathway and is now in clinical trials VSports手机版. We and others have previously shown that the antitumor activity of MLN4924 is mediated by its ability to induce apoptosis, autophagy and senescence in a cell context-dependent manner. However, whether MLN4924 has any effect on tumor angiogenesis remains unexplored. Here we report that MLN4924 inhibits angiogenesis in various in vitro and in vivo models, leading to the suppression of tumor growth and metastasis in highly malignant pancreatic cancer, indicating that blockage of angiogenesis is yet another mechanism contributing to its antitumor activity. At the molecular level, MLN4924 inhibits Cullin-RING E3 ligases (CRLs) by cullin deneddylation, causing accumulation of RhoA at an early stage to impair angiogenic activity of vascular endothelial cells and subsequently DNA damage response, cell cycle arrest and apoptosis due to accumulation of other tumor-suppressive substrates of CRLs. Furthermore, we showed that inactivation of CRLs, via small interfering RNA (siRNA) silencing of its essential subunit ROC1/RBX1, recapitulates the antiangiogenic effect of MLN4924. Taken together, our study demonstrates a previously unrecognized role of neddylation in the regulation of tumor angiogenesis using both pharmaceutical and genetic approaches, and provides proof of concept evidence for future development of neddylation inhibitors (such as MLN4924) as a novel class of antiangiogenic agents. .
Figures
References
-
- Xirodimas DP. Novel substrates and functions for the ubiquitin-like molecule NEDD8. Biochem Soc Trans. 2008;36 (Pt 5:802–806. - PubMed
-
- Sakata E, Yamaguchi Y, Miyauchi Y, Iwai K, Chiba T, Saeki Y, et al. Direct interactions between NEDD8 and ubiquitin E2 conjugating enzymes upregulate cullin-based E3 ligase activity. Nat Struct Mol Biol. 2007;14:167–168. - PubMed
-
- Wu JT, Lin HC, Hu YC, Chien CT. Neddylation and deneddylation regulate Cul1 and Cul3 protein accumulation. Nat Cell Biol. 2005;7:1014–1020. - PubMed
-
- Nalepa G, Rolfe M, Harper JW. Drug discovery in the ubiquitin-proteasome system. Nat Rev Drug Discov. 2006;5:596–613. - "V体育平台登录" PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- Actions (VSports注册入口)
- "VSports注册入口" Actions
- Actions (V体育2025版)
- Actions (VSports手机版)
- "V体育官网入口" Actions
- "V体育ios版" Actions
- Actions (V体育官网入口)
- "V体育官网入口" Actions
- Actions (V体育2025版)
- "V体育官网" Actions
- Actions (VSports在线直播)
- Actions (VSports)
- VSports在线直播 - Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- "V体育官网" Actions
- VSports注册入口 - Actions
- "V体育安卓版" Actions
- Actions (V体育官网)
- Actions (V体育平台登录)
- "V体育安卓版" Actions
Substances
- V体育官网入口 - Actions
- V体育平台登录 - Actions
- Actions (V体育官网入口)
- Actions (VSports注册入口)
- V体育安卓版 - Actions
- Actions (V体育官网)
- VSports手机版 - Actions
- Actions (V体育官网)
LinkOut - more resources
Full Text Sources
Other Literature Sources (V体育平台登录)
Medical
Miscellaneous

