Discrepant NOXA (PMAIP1) transcript and NOXA protein levels: a potential Achilles' heel in mantle cell lymphoma
- PMID: 24457957
- PMCID: PMC4040662
- DOI: 10.1038/cddis.2013.552
Discrepant NOXA (PMAIP1) transcript and NOXA protein levels: a potential Achilles' heel in mantle cell lymphoma
Abstract (VSports)
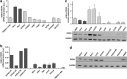
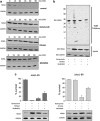
Mantle cell lymphoma (MCL) is an aggressive lymphoid neoplasm with transient response to conventional chemotherapy. We here investigated the role of the Bcl-2 homology domain 3-only protein NOXA for life-death decision in MCL. Surprisingly, NOXA (PMAIP1) mRNA and NOXA protein levels were extremely discrepant in MCL cells: NOXA mRNA was found to be highly expressed whereas NOXA protein levels were low. Chronic active B-cell receptor signaling and to a minor degree cyclin D1 overexpression contributed to high NOXA mRNA expression levels in MCL cells. The phoshatidyl-inositol-3 kinase/AKT/mammalian target of rapamycin pathway was identified as the major downstream signaling pathway involved in the maintenance of NOXA gene expression. Interestingly, MCL cells adapt to this constitutive pro-apoptotic signal by extensive ubiquitination and rapid proteasomal degradation of NOXA protein (T½∼15-30 min). In addition to the proteasome inhibitor Bortezomib, we identified the neddylation inhibitor MLN4924 and the fatty acid synthase inhibitor Orlistat as potent inducers of NOXA protein expression leading to apoptosis in MCL VSports手机版. All inhibitors targeted NOXA protein turnover. In contrast to Bortezomib, MLN4924 and Orlistat interfered with the ubiquitination process of NOXA protein thereby offering new strategies to kill Bortezomib-resistant MCL cells. Our data, therefore, highlight a critical role of NOXA in the balance between life and death in MCL. The discrepancy between NOXA transcript and protein levels is essential for sensitivity of MCL to ubiquitin-proteasome system inhibitors and could therefore provide a druggable Achilles' heel of MCL cells. .
V体育2025版 - Figures



References
-
- Jares P, Colomer D, Campo E. Genetic and molecular pathogenesis of mantle cell lymphoma: perspectives for new targeted therapeutics. Nat Rev Cancer. 2007;7:750–762. - PubMed
-
- Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122:3416–3423. - PMC (V体育平台登录) - PubMed
-
- Pérez-Galán P, Dreyling M, Wiestner A. Mantle cell lymphoma: biology, pathogenesis, and the molecular basis of treatment in the genomic era. Blood. 2011;117:26–38. - PMC (VSports手机版) - PubMed
-
- Fernàndez V, Hartmann E, Ott G, Campo E, Rosenwald A. Pathogenesis of mantle-cell lymphoma: all oncogenic roads lead to dysregulation of cell cycle and DNA damage response pathways. J Clin Oncol. 2005;23:6364–6369. - PubMed
-
- Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12:229–243. - PMC (VSports) - PubMed
MeSH terms
- "V体育官网" Actions
- VSports最新版本 - Actions
- "V体育ios版" Actions
- Actions (VSports最新版本)
- V体育官网入口 - Actions
- VSports手机版 - Actions
- "VSports手机版" Actions
- VSports手机版 - Actions
Substances
- Actions (VSports在线直播)
- VSports app下载 - Actions
- Actions (VSports app下载)
LinkOut - more resources (VSports在线直播)
Full Text Sources
Other Literature Sources
Research Materials

