V体育平台登录 - P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions
- PMID: 24448686
- PMCID: PMC3989397
- DOI: 10.1158/1541-7786.MCR-13-0489
P-cadherin promotes ovarian cancer dissemination through tumor cell aggregation and tumor-peritoneum interactions
Abstract
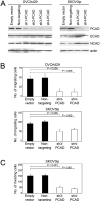
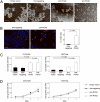
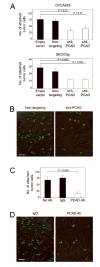
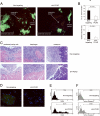
More than 60% of patients who are diagnosed with epithelial ovarian cancer (EOC) present with extensive peritoneal carcinomatosis. EOC cells typically disseminate by shedding into the peritoneal fluid in which they survive as multicellular aggregates and then implant onto peritoneal surfaces. However, the mechanism that facilitates aggregation and implantation of EOC cells is poorly understood VSports手机版. The cell adhesion molecule P-cadherin has been reported to be induced during early progression of EOC and to promote tumor cell migration. In this study, P-cadherin not only promoted migration of EOC cells, but also facilitated the assembly of floating EOC cells into multicellular aggregates and inhibited anoikis in vitro. Furthermore, inhibiting P-cadherin by short hairpin RNAs (shRNA) or a neutralizing antibody prevented EOC cells from attaching to peritoneal mesothelial cells in vitro. In mouse intraperitoneal xenograft models of EOC, inhibition of P-cadherin decreased the aggregation and survival of floating tumor cells in ascites and reduced the number of tumor implants on peritoneal surfaces. These findings indicate that P-cadherin promotes intraperitoneal dissemination of EOC by facilitating tumor cell aggregation and tumor-peritoneum interactions in addition to promoting tumor cell migration. .
Implications: Inhibiting P-cadherin blocks multiple key steps of EOC progression and has therapeutic potential V体育安卓版. .
Mol Cancer Res; 12(4); 504-13 V体育ios版. ©2014 AACR. .
V体育官网 - Figures

References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Naora H, Montell DJ. Ovarian cancer metastasis: Integrating insights from disparate model organisms. Nat Rev Cancer. 2005;5:355–66. - PubMed
-
- Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegama TR, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–81. - PubMed
Publication types
"V体育官网" MeSH terms
- Actions (V体育安卓版)
- VSports手机版 - Actions
- V体育官网入口 - Actions
- "V体育2025版" Actions
- Actions (VSports在线直播)
- VSports - Actions
- "V体育ios版" Actions
- V体育安卓版 - Actions
Substances
- Actions (V体育2025版)
Grants and funding (VSports app下载)
LinkOut - more resources
Full Text Sources
"VSports最新版本" Other Literature Sources
Medical
Research Materials

