Rapid induction of alternative lengthening of telomeres by depletion of the histone chaperone ASF1
- PMID: 24413054
- PMCID: PMC3946341
- DOI: 10.1038/nsmb.2754
Rapid induction of alternative lengthening of telomeres by depletion of the histone chaperone ASF1
Abstract
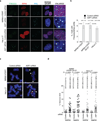
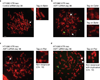
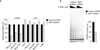
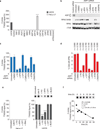
The mechanism of activation of the alternative lengthening of telomeres (ALT) pathway of mammalian chromosome-end maintenance has been unclear. We have now discovered that co-depletion of the histone chaperones ASF1a and ASF1b in human cells induced all hallmarks of ALT in both primary and cancer cells. These included the formation of ALT-associated PML (promyelocytic leukemia) bodies (APBs), the presence of extrachromosomal telomeric DNA species, an elevated frequency of telomeric sister chromatid exchanges (t-SCE) events and intertelomeric exchange of an integrated tag. The induction of ALT characteristics in this setting led to the simultaneous suppression of telomerase. We determined that ALT induction is positively regulated by the proteins RAD17 and BLM and negatively regulated by EXO1 and DNA2. The induction of ALT phenotypes as a consequence of ASF1 depletion strongly supports the hypothesis that ALT is a consequence of histone management dysfunction VSports手机版. .
Figures



"V体育平台登录" References
-
- Cesare AJ, Reddel RR. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. - "VSports" PubMed
-
- Heaphy CMC, et al. Prevalence of the alternative lengthening of telomeres telomere maintenance mechanism in human cancer subtypes. Am J Pathol. 2011;179:1608–1615. - "V体育官网" PMC - PubMed
-
- Henson JD, Reddel RR. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Letters. 2010;584:3800–3811. - PubMed
-
- Londoño-Vallejo JA, Der-Sarkissian H, Cazes L, Bacchetti S, Reddel RR. Alternative lengthening of telomeres is characterized by high rates of telomeric exchange. Cancer Res. 2004;64:2324–2327. - VSports注册入口 - PubMed
-
- Yeager TRT, et al. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 1999;59:4175–4179. - PubMed
Publication types
- VSports最新版本 - Actions
MeSH terms
- V体育官网入口 - Actions
- "VSports注册入口" Actions
Substances
- V体育安卓版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

