c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells
- PMID: 24394663
- PMCID: PMC3924926
- DOI: 10.1182/blood-2013-08-522698
c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells
Abstract
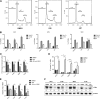
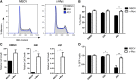
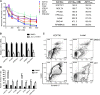
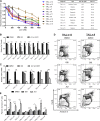
Although prognosis has improved for children with T-cell acute lymphoblastic leukemia (T-ALL), 20% to 30% of patients undergo induction failure (IF) or relapse. Leukemia-initiating cells (LICs) are hypothesized to be resistant to chemotherapy and to mediate relapse. We and others have shown that Notch1 directly regulates c-Myc, a known regulator of quiescence in stem and progenitor populations, leading us to examine whether c-Myc inhibition results in efficient targeting of T-ALL-initiating cells. We demonstrate that c-Myc suppression by small hairpin RNA or pharmacologic approaches prevents leukemia initiation in mice by eliminating LIC activity. Consistent with its anti-LIC activity in mice, treatment with the BET bromodomain BRD4 inhibitor JQ1 reduces C-MYC expression and inhibits the growth of relapsed and IF pediatric T-ALL samples in vitro. These findings demonstrate a critical role for c-Myc in LIC maintenance and provide evidence that MYC inhibition may be an effective therapy for relapsed/IF T-ALL patients. VSports手机版.
Figures


References
-
- Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–271. - PubMed
-
- Malyukova A, Dohda T, von der Lehr N, et al. The tumor suppressor gene hCDC4 is frequently mutated in human T-cell acute lymphoblastic leukemia with functional consequences for Notch signaling. Cancer Res. 2007;67(12):5611–5616. - PubMed
-
- Thompson BJ, Buonamici S, Sulis ML, et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J Exp Med. 2007;204(8):1825–1835. - VSports最新版本 - PMC - PubMed
Publication types
MeSH terms (V体育官网)
- Actions (V体育ios版)
- VSports注册入口 - Actions
- Actions (V体育ios版)
- "V体育官网入口" Actions
- V体育ios版 - Actions
- "V体育官网入口" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
- VSports app下载 - Actions
- V体育官网入口 - Actions
- Actions (V体育安卓版)
- "VSports手机版" Actions
- "V体育官网入口" Actions
- "VSports注册入口" Actions
- "VSports app下载" Actions
Substances
- Actions (VSports app下载)
Grants and funding (VSports在线直播)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous (VSports app下载)

