Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization
- PMID: 24332030
- PMCID: "VSports注册入口" PMC3886715
- DOI: 10.1016/j.immuni.2013.10.019 (VSports)
Cyclic GMP-AMP synthase is activated by double-stranded DNA-induced oligomerization
Abstract
Cyclic GMP-AMP synthase (cGAS) is a cytosolic DNA sensor mediating innate antimicrobial immunity VSports手机版. It catalyzes the synthesis of a noncanonical cyclic dinucleotide, 2',5' cGAMP, that binds to STING and mediates the activation of TBK1 and IRF-3. Activated IRF-3 translocates to the nucleus and initiates the transcription of the IFN-β gene. The structure of mouse cGAS bound to an 18 bp dsDNA revealed that cGAS interacts with dsDNA through two binding sites, forming a 2:2 complex. Enzyme assays and IFN-β reporter assays of cGAS mutants demonstrated that interactions at both DNA binding sites are essential for cGAS activation. Mutagenesis and DNA binding studies showed that the two sites bind dsDNA cooperatively and that site B plays a critical role in DNA binding. The structure of mouse cGAS bound to dsDNA and 2',5' cGAMP provided insight into the catalytic mechanism of cGAS. These results demonstrated that cGAS is activated by dsDNA-induced oligomerization. .
Copyright © 2013 Elsevier Inc V体育安卓版. All rights reserved. .
"VSports最新版本" Figures



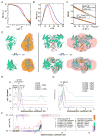
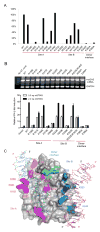

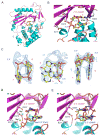
Comment in
-
cGAS dimerization entangles DNA recognition.Immunity. 2013 Dec 12;39(6):992-4. doi: 10.1016/j.immuni.2013.11.012. Immunity. 2013. PMID: 24332024
References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. - VSports - PMC - PubMed
-
- Barber GN. Innate immune DNA sensing pathways: STING, AIMII and the regulation of interferon production and inflammatory responses. Curr Opin Immunol. 2011a;23:10–20. - "V体育ios版" PMC - PubMed
-
- Barber GN. STING-dependent signaling. Nat Immunol. 2011b;12:929–930. - PubMed
Publication types
- Actions (V体育官网入口)
MeSH terms
- V体育平台登录 - Actions
- V体育ios版 - Actions
- Actions (VSports手机版)
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- "VSports最新版本" Actions
- "VSports最新版本" Actions
- VSports app下载 - Actions
Substances (V体育官网)
- "V体育官网" Actions
- "VSports手机版" Actions
- V体育ios版 - Actions
Associated data
- Actions
- VSports最新版本 - Actions
- VSports手机版 - Actions
- Actions (V体育官网入口)
"V体育官网入口" Grants and funding
LinkOut - more resources
Full Text Sources (V体育2025版)
Other Literature Sources
VSports app下载 - Molecular Biology Databases
Research Materials
Miscellaneous (VSports在线直播)

