Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype
- PMID: 24332016
- PMCID: VSports手机版 - PMC3873497
- DOI: VSports app下载 - 10.1016/j.ajpath.2013.09.017
"V体育官网" Expression of the homeobox gene HOXA9 in ovarian cancer induces peritoneal macrophages to acquire an M2 tumor-promoting phenotype
"V体育ios版" Abstract
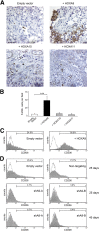
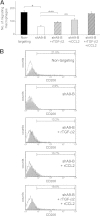
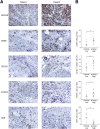
Tumor-associated macrophages (TAMs) exhibit an M2 macrophage phenotype that suppresses anti-tumor immune responses and often correlates with poor outcomes in patients with cancer. Patients with ovarian cancer frequently present with peritoneal carcinomatosis, but the mechanisms that induce naïve peritoneal macrophages into TAMs are poorly understood. In this study, we found an increased abundance of TAMs in mouse i. p. xenograft models of ovarian cancer that expressed HOXA9, a homeobox gene that is associated with poor prognosis in patients with ovarian cancer VSports手机版. HOXA9 expression in ovarian cancer cells stimulated chemotaxis of peritoneal macrophages and induced macrophages to acquire TAM-like features. These features included induction of the M2 markers, CD163 and CD206, and the immunosuppressive cytokines, IL-10 and chemokine ligand 17, and down-regulation of the immunostimulatory cytokine, IL-12. HOXA9-mediated induction of TAMs was primarily due to the combinatorial effects of HOXA9-induced, tumor-derived transforming growth factor-β2 and chemokine ligand 2 levels. High HOXA9 expression in clinical specimens of ovarian cancer was strongly associated with increased abundance of TAMs and intratumoral T-regulatory cells and decreased abundance of CD8(+) tumor-infiltrating lymphocytes. Levels of immunosuppressive cytokines were also elevated in ascites fluid of patients with tumors that highly expressed HOXA9. HOXA9 may, therefore, stimulate ovarian cancer progression by promoting an immunosuppressive microenvironment via paracrine effects on peritoneal macrophages. .
Copyright © 2014 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved V体育安卓版. .
Figures



References
-
- Tlsty T.D., Coussens L.M. Tumor stroma and regulation of cancer development. Annu Rev Pathol. 2006;1:119–150. - PubMed
-
- Kalluri R., Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer. 2006;6:392–401. - PubMed
-
- Mantovani A., Sozzani S., Locati M., Allavena P., Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. - PubMed
-
- Solinas G., Germano G., Mantovani A., Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. - PubMed
-
- Pollard J.W. Tumor-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer. 2004;4:71–78. - "VSports" PubMed
Publication types
"VSports手机版" MeSH terms
- Actions (VSports app下载)
- Actions (VSports在线直播)
- "VSports最新版本" Actions
- "V体育官网入口" Actions
- "VSports在线直播" Actions
- VSports注册入口 - Actions
- "VSports注册入口" Actions
- "VSports最新版本" Actions
- "V体育ios版" Actions
- Actions (VSports)
- "V体育安卓版" Actions
Substances (V体育ios版)
- VSports手机版 - Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical (VSports最新版本)
VSports - Research Materials

