Klotho has dual protective effects on cisplatin-induced acute kidney injury (V体育官网入口)
- PMID: 24304882
- PMCID: PMC3972320
- DOI: 10.1038/ki.2013.489
Klotho has dual protective effects on cisplatin-induced acute kidney injury
"V体育2025版" Abstract
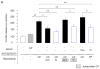
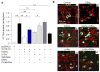
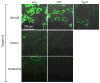
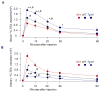
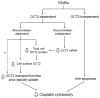
Klotho protects the kidney from ischemia-reperfusion injury, but its effect on nephrotoxins is unknown. Here we determined whether Klotho protects the kidney from cisplatin toxicity VSports手机版. Cisplatin increased plasma creatinine and induced tubular injury, which were exaggerated in Klotho haplosufficient (Kl/+) and ameliorated in transgenic Klotho overexpressing (Tg-Kl) mice. Neutrophil gelatinase-associated lipocalin and active caspase-3 protein and the number of apoptotic cells in the kidney were higher in Kl/+ and lower in Tg-Kl compared with wild-type mice. Klotho suppressed basolateral uptake of cisplatin by the normal rat kidney cell line (NRK), an effect similar to cimetidine, a known inhibitor of organic cation transport (OCT). A decrease in cell surface and total OCT2 protein and OCT activity by Klotho was mimicked by β-glucuronidase. The Klotho effect was attenuated by β-glucuronidase inhibition. On the other hand, OCT2 mRNA was reduced by Klotho but not by β-glucuronidase. Moreover, cimetidine inhibited OCT activity but not OCT2 expression. Unlike cimetidine, Klotho reduced cisplatin-induced apoptosis from either the basolateral or apical side and even when added after NRK cells were already loaded with cisplatin. Thus, Klotho protects the kidney against cisplatin nephrotoxicity by reduction of basolateral uptake of cisplatin by OCT2 and a direct anti-apoptotic effect independent of cisplatin uptake. Klotho may be a useful agent to prevent and treat cisplatin-induced nephrotoxicity. .
"VSports在线直播" Figures
























References
-
- Lippman AJ, Helson C, Helson L, et al. Clinical trials of cis-diamminedichloroplatinum (NSC-119875) Cancer Chemother Rep. 1973;57:191–200. - PubMed
-
- Hardaker WT, Jr, Stone RA, McCoy R. Platinum nephrotoxicity. Cancer. 1974;34:1030–1032. - PubMed
-
- Gaspari F, Cravedi P, Mandala M, et al. Predicting cisplatin-induced acute kidney injury by urinary neutrophil gelatinase-associated lipocalin excretion: a pilot prospective case-control study. Nephron Clin Pract. 2010;115:c154–160. - PubMed
-
- Launay-Vacher V, Rey JB, Isnard-Bagnis C, et al. Prevention of cisplatin nephrotoxicity: state of the art and recommendations from the European Society of Clinical Pharmacy Special Interest Group on Cancer Care. Cancer Chemother Pharmacol. 2008;61:903–909. - PubMed
-
- Yao X, Panichpisal K, Kurtzman N, et al. Cisplatin nephrotoxicity: a review. Am J Med Sci. 2007;334:115–124. - PubMed
V体育ios版 - Publication types
- V体育ios版 - Actions
MeSH terms
- "VSports最新版本" Actions
- "V体育ios版" Actions
- Actions (V体育2025版)
- Actions (VSports app下载)
- Actions (V体育ios版)
- Actions (V体育2025版)
- V体育官网入口 - Actions
- V体育安卓版 - Actions
- "VSports" Actions
Substances
- "VSports app下载" Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
"VSports手机版" Miscellaneous

