3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome
- PMID: 24265316
- PMCID: "VSports app下载" PMC3887181
- DOI: VSports最新版本 - 10.1074/jbc.M113.515080
V体育ios版 - 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome
Abstract
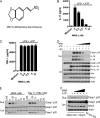
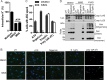
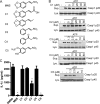
The NLRP3 inflammasome is a critical component of the innate immune system VSports手机版. NLRP3 activation is induced by diverse stimuli associated with bacterial infection or tissue damage, but its inappropriate activation is involved in the pathogenesis of inherited and acquired inflammatory diseases. However, the mechanism by which NLRP3 is activated remains poorly understood. In this study, we explored the role of kinases in NLRP3 inflammasome activation by screening a kinase inhibitor library and identified 3,4-methylenedioxy-β-nitrostyrene (MNS) as an inhibitor for NLRP3 inflammasome activation. Notably, MNS did not affect the activation of the NLRC4 or AIM2 (absent in melanoma 2) inflammasome. Mechanistically, MNS specifically prevented NLRP3-mediated ASC speck formation and oligomerization without blocking potassium efflux induced by NLRP3 agonists. Surprisingly, Syk kinase, the reported target of MNS, did not mediate the inhibitory activity of MNS on NLRP3 inflammasome activation. We also found that the nitrovinyl group of MNS is essential for the inhibitory activity of MNS. Immunoprecipitation, mass spectrometry, and mutation studies suggest that both the nucleotide binding oligomerization domain and the leucine-rich repeat domain of NLRP3 were the intracellular targets of MNS. Administration of MNS also inhibited NLRP3 ATPase activity in vitro, suggesting that MNS blocks the NLRP3 inflammasome by directly targeting NLRP3 or NLRP3-associated complexes. These studies identified a novel chemical probe for studying the molecular mechanism of NLRP3 inflammasome activation which may advance the development of novel strategies to treat diseases associated with abnormal activation of NLRP3 inflammasome. .
Keywords: Caspase; Inflammation; Innate Immunity; Macrophages; NOD-like Receptor (NLR) V体育安卓版. .
"VSports在线直播" Figures
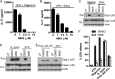


References
-
- Dinarello C. A. (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 - "VSports app下载" PubMed
-
- Martinon F., Mayor A., Tschopp J. (2009) The inflammasomes: guardians of the body. Annu. Rev. Immunol. 27, 229–265 - PubMed
-
- Franchi L., Muñoz-Planillo R., Núñez G. (2012) Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 13, 325–332 - "VSports最新版本" PMC - PubMed
-
- Schroder K., Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 - PubMed
Publication types
MeSH terms
- "VSports app下载" Actions
- V体育官网入口 - Actions
- Actions (V体育官网入口)
- "V体育安卓版" Actions
- "V体育安卓版" Actions
- V体育官网 - Actions
- "VSports app下载" Actions
- "VSports" Actions
- V体育安卓版 - Actions
- Actions (V体育ios版)
- V体育官网入口 - Actions
- "V体育平台登录" Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- VSports在线直播 - Actions
- "V体育ios版" Actions
Substances
- "VSports app下载" Actions
- "V体育2025版" Actions
- Actions (VSports注册入口)
- V体育官网入口 - Actions
- Actions (V体育ios版)
- "V体育安卓版" Actions
"VSports最新版本" Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

