Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation
- PMID: 24226773
- PMCID: PMC3869884 (V体育平台登录)
- DOI: 10.1038/nature12726
V体育平台登录 - Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation
Abstract
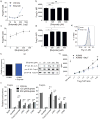
Intestinal microbes provide multicellular hosts with nutrients and confer resistance to infection. The delicate balance between pro- and anti-inflammatory mechanisms, essential for gut immune homeostasis, is affected by the composition of the commensal microbial community. Regulatory T cells (Treg cells) expressing transcription factor Foxp3 have a key role in limiting inflammatory responses in the intestine. Although specific members of the commensal microbial community have been found to potentiate the generation of anti-inflammatory Treg or pro-inflammatory T helper 17 (TH17) cells, the molecular cues driving this process remain elusive. Considering the vital metabolic function afforded by commensal microorganisms, we reasoned that their metabolic by-products are sensed by cells of the immune system and affect the balance between pro- and anti-inflammatory cells. We tested this hypothesis by exploring the effect of microbial metabolites on the generation of anti-inflammatory Treg cells. We found that in mice a short-chain fatty acid (SCFA), butyrate, produced by commensal microorganisms during starch fermentation, facilitated extrathymic generation of Treg cells VSports手机版. A boost in Treg-cell numbers after provision of butyrate was due to potentiation of extrathymic differentiation of Treg cells, as the observed phenomenon was dependent on intronic enhancer CNS1 (conserved non-coding sequence 1), essential for extrathymic but dispensable for thymic Treg-cell differentiation. In addition to butyrate, de novo Treg-cell generation in the periphery was potentiated by propionate, another SCFA of microbial origin capable of histone deacetylase (HDAC) inhibition, but not acetate, which lacks this HDAC-inhibitory activity. Our results suggest that bacterial metabolites mediate communication between the commensal microbiota and the immune system, affecting the balance between pro- and anti-inflammatory mechanisms. .
Figures


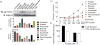
Comment in
-
Mucosal immunology: Bacteria get T(Reg) cells into shape. (VSports)Nat Rev Immunol. 2014 Jan;14(1):2-3. doi: 10.1038/nri3583. Epub 2013 Nov 29. Nat Rev Immunol. 2014. PMID: 24287865 No abstract available.
References
-
- Josefowicz SZ, et al. Extrathymically generated regulatory T cells control mucosal T(H)2 inflammation. Nature. 2012;482:395–U1510. - "VSports在线直播" PMC - PubMed
-
- Round JL, Mazmanian SK. Inducible Foxp (3+) regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12204–12209. - "V体育ios版" PMC - PubMed
-
- Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;13998:485. - "VSports手机版" PMC - PubMed
Publication types
- Actions (VSports在线直播)
"V体育官网入口" MeSH terms
- Actions (VSports在线直播)
- Actions (V体育官网)
- Actions (VSports最新版本)
- VSports - Actions
- Actions (V体育官网入口)
- V体育2025版 - Actions
- "VSports注册入口" Actions
- "V体育安卓版" Actions
- V体育官网入口 - Actions
- "V体育官网入口" Actions
- "V体育官网入口" Actions
- Actions (VSports手机版)
- VSports最新版本 - Actions
- VSports在线直播 - Actions
- VSports注册入口 - Actions
- V体育平台登录 - Actions
- "V体育平台登录" Actions
- "VSports" Actions
VSports注册入口 - Substances
- V体育官网入口 - Actions
- "V体育官网入口" Actions
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

