Endothelial deletion of Sag/Rbx2/Roc2 E3 ubiquitin ligase causes embryonic lethality and blocks tumor angiogenesis
- PMID: 24213570
- PMCID: PMC4016996
- DOI: "VSports" 10.1038/onc.2013.473
Endothelial deletion of Sag/Rbx2/Roc2 E3 ubiquitin ligase causes embryonic lethality and blocks tumor angiogenesis
Abstract
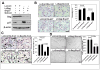
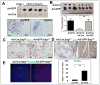
SAG (Sensitive to Apoptosis Gene), also known as RBX2 or ROC2, is a RING protein required for the activity of Cullin-RING ligase (CRL) VSports手机版. Our recent study showed that Sag total knockout caused embryonic lethality at E11. 5-12. 5 days with associated defects in vasculogenesis. Whether Sag is required for de novo vasculogenesis in embryos and angiogenesis in tumors is totally unknown. Here, we report that Sag endothelial deletion also causes embryonic lethality at E15. 5 with poor vasculogenesis. Sag deletion in primary endothelial cells (ECs) or knockdown in MS-1 ECs inhibits migration, proliferation and tube formation, with p27 accumulation being responsible for the suppression of migration and proliferation. Furthermore, Sag deletion significantly inhibits angiogenesis in an in vivo Matrigel plug assay, and tumor angiogenesis and tumorigenesis in a B16F10 melanoma model. Finally, MLN4924, an investigational small molecule inhibitor of NEDD8-activating enzyme (NAE) that inhibits CRL, suppresses in vitro migration, proliferation and tube formation, as well as in vivo angiogenesis and tumorigenesis. Taken together, our study, using both genetic and pharmaceutical approaches, demonstrates that Sag is essential for embryonic vasculogenesis and tumor angiogenesis, and provides the proof-of-concept evidence that targeting Sag E3 ubiquitin ligase may have clinical value for anti-angiogenesis therapy of human cancer. .
"V体育官网入口" Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. - PubMed
-
- Nakayama KI, Nakayama K. Ubiquitin ligases: cell-cycle control and cancer. Nat Rev Cancer. 2006;6:369–381. - PubMed
-
- Zhao Y, Sun Y. Cullin-RING Ligases as Attractive Anti-cancer Targets. Curr Pharm Des. 2013;19:3215–3225. - PMC (V体育2025版) - PubMed
Publication types
- "V体育2025版" Actions
MeSH terms
- VSports app下载 - Actions
- V体育安卓版 - Actions
- VSports app下载 - Actions
- VSports手机版 - Actions
- "VSports app下载" Actions
- VSports app下载 - Actions
- "VSports app下载" Actions
- "V体育官网" Actions
- "V体育ios版" Actions
- Actions (VSports app下载)
- VSports最新版本 - Actions
Substances
- Actions (VSports最新版本)
- Actions (VSports)
- "VSports手机版" Actions
- Actions (VSports)
- Actions (VSports)
Grants and funding
"V体育平台登录" LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

