V体育安卓版 - Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain
- PMID: 24167251
- PMCID: PMC3831961
- DOI: "VSports最新版本" 10.1073/pnas.1311749110
Ctr2 regulates biogenesis of a cleaved form of mammalian Ctr1 metal transporter lacking the copper- and cisplatin-binding ecto-domain
Abstract
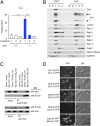
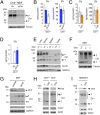
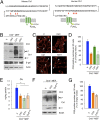
Copper is an essential catalytic cofactor for enzymatic activities that drive a range of metabolic biochemistry including mitochondrial electron transport, iron mobilization, and peptide hormone maturation. Copper dysregulation is associated with fatal infantile disease, liver, and cardiac dysfunction, neuropathy, and anemia. Here we report that mammals regulate systemic copper acquisition and intracellular mobilization via cleavage of the copper-binding ecto-domain of the copper transporter 1 (Ctr1). Although full-length Ctr1 is critical to drive efficient copper import across the plasma membrane, cleavage of the ecto-domain is required for Ctr1 to mobilize endosomal copper stores VSports手机版. The biogenesis of the truncated form of Ctr1 requires the structurally related, previously enigmatic copper transporter 2 (Ctr2). Ctr2(-/-) mice are defective in accumulation of truncated Ctr1 and exhibit increased tissue copper levels, and X-ray fluorescence microscopy demonstrates that copper accumulates as intracellular foci. These studies identify a key regulatory mechanism for mammalian copper transport through Ctr2-dependent accumulation of a Ctr1 variant lacking the copper- and cisplatin-binding ecto-domain. .
Keywords: endosome; lysosome; platinum; protein regulation; uptake. V体育安卓版.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
"VSports最新版本" Publication types
VSports app下载 - MeSH terms
- Actions (VSports注册入口)
- Actions (VSports在线直播)
- Actions (VSports app下载)
- "V体育平台登录" Actions
- "V体育安卓版" Actions
- Actions (VSports)
VSports最新版本 - Substances
- Actions (V体育安卓版)
- Actions (VSports最新版本)
V体育安卓版 - Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
VSports注册入口 - Research Materials

