Cu/Nitroxyl Catalyzed Aerobic Oxidation of Primary Amines into Nitriles at Room Temperature (V体育官网)
- PMID: 24015373
- PMCID: PMC3763855
- DOI: V体育官网 - 10.1021/cs400360e
Cu/Nitroxyl Catalyzed Aerobic Oxidation of Primary Amines into Nitriles at Room Temperature
Abstract
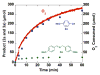
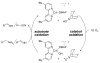
An efficient catalytic method has been developed for aerobic oxidation of primary amines to the corresponding nitriles. The reactions proceed at room temperature and employ a catalyst consisting of (4,4'- t Bu2bpy)CuI/ABNO (ABNO = 9-azabicyclo[3. 3. 1]nonan-3-one N-oxyl). The reactions exhibit excellent functional group compatibility and substrate scope, and are effective with benzylic, allylic and aliphatic amines VSports手机版. Preliminary mechanistic studies suggest that aerobic oxidation of the Cu catalyst is the turnover-limiting step of the reaction. .
Keywords: aerobic oxidation; amine oxidation; copper; nitrile; nitroxyl. V体育安卓版.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
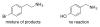


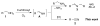
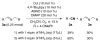
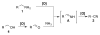
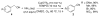
References
-
-
For reviews of aerobic alcohol oxidation, see: Arends IWCE, Sheldon RA. In: In Modern Oxidation Methods. Bäckvall JE, editor. Wiley-VCH Verlag Gmb & Co.; Weinheim: 2004. pp. 83–118.Sheldon RA, Arends IWCE, ten Brink GJ, Dijksman A. Acc Chem Res. 2002;35:774.Schultz MJ, Sigman MS. Tetrahedron. 2006;62:8227.Parmeggiani C, Cardona F. Green Chem. 2012;14:547.
-
-
-
For a review of aerobic oxidation of amines, see: Schümperli MT, Hammond C, Hermans I. ACS Catal. 2012;2:1108.
-
-
- Brackman W, Gaasbeek C. Rec Trav Chim. 1966;85:221.
- Brackman W, Gaasbeek C. Rec Trav Chim. 1966;85:257.
-
-
For leading references to Cu/TEMPO-mediated aerobic alcohol oxidation, see the following: Semmelhack MF, Schmid CR, Cortés DA, Chou CS. J Am Chem Soc. 1984;106:3374.Ragagnin G, Betzemeier B, Quici S, Knochel P. Tetrahedron. 2002;58:3985.Gamez P, Arends IWCE, Reedijk J, Sheldon RA. Chem Commun. 2003:2414.Gamez P, Arends IWCE, Sheldon RA, Reedijk J. Adv Synth Catal. 2004;346:805.Geisslmeir D, Jary WG, Falk H. Monatsh Chem. 2005;136:1591.Jiang N, Ragauskas AJ. J Org Chem. 2006;71:7087.Mannam S, Alamsetti SK, Sekar G. Adv Synth Catal. 2007;349:2253.Kumpulainen ETT, Koskinen AMP. Chem Eur J. 2009;15:10901.Hoover JM, Stahl SS. J Am Chem Soc. 2011;133:16901.Hoover JM, Steves JE, Stahl SS. Nat Protoc. 2012;7:1161.Könning D, Hiller W, Christmann M. Org Lett. 2012;14:5258.
-
-
-
For leading references to Cu/TEMPO and related catalyst systems for amine oxidation, see the following: Han B, Yang XL, Wang C, Bai YW, Pan TC, Chen X, Yu W. J Org Chem. 2012;77:1136.Hu (VSports在线直播) Z, Kerton FM. Org Biomol Chem. 2012;10:1618.Sonobe T, Oisaki K, Kanai M. Chem Sci. 2012;3:3249.Huang B, Tian H, Lin S, Xie M, Yu X, Xu Q. Tetrahedron Lett. 2013;54:2861.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
