Modification of Akt by SUMO conjugation regulates alternative splicing and cell cycle (V体育安卓版)
- PMID: 24013425
- PMCID: "VSports注册入口" PMC3865012
- DOI: 10.4161/cc.26183 (VSports最新版本)
Modification of Akt by SUMO conjugation regulates alternative splicing and cell cycle
Abstract
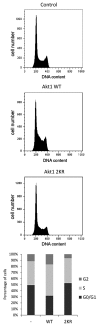
Akt/PKB is a key signaling molecule in higher eukaryotes and a crucial protein kinase in human health and disease. Phosphorylation, acetylation, and ubiquitylation have been reported as important regulatory post-translational modifications of this kinase VSports手机版. We describe here that Akt is modified by SUMO conjugation, and show that lysine residues 276 and 301 are the major SUMO attachment sites within this protein. We found that phosphorylation and SUMOylation of Akt appear as independent events. However, decreasing Akt SUMOylation levels severely affects the role of this kinase as a regulator of fibronectin and Bcl-x alternative splicing. Moreover, we observed that the Akt mutant (Akt E17K) found in several human tumors displays increased levels of SUMOylation and also an enhanced capacity to regulate fibronectin splicing patterns. This splicing regulatory activity is completely abolished by decreasing Akt E17K SUMO conjugation levels. Additionally, we found that SUMOylation controls Akt regulatory function at G₁/S transition during cell cycle progression. These findings reveal SUMO conjugation as a novel level of regulation for Akt activity, opening new areas of exploration related to the molecular mechanisms involved in the diverse cellular functions of this kinase. .
Keywords: Akt/PKB; SUMO; alternative splicing; cell cycle; post-translational modification; signal transduction. V体育安卓版.
"VSports在线直播" Figures




"V体育安卓版" References
-
- Hochstrasser M. SP-RING for SUMO: new functions bloom for a ubiquitin-like protein. Cell. 2001;107:5–8. doi: 10.1016/S0092-8674(01)00519-0. - "V体育平台登录" DOI - PubMed
Publication types
MeSH terms
- "VSports app下载" Actions
- "V体育ios版" Actions
- "VSports" Actions
- "V体育2025版" Actions
- "V体育官网" Actions
- Actions (V体育平台登录)
- "V体育2025版" Actions
- Actions (V体育2025版)
- "VSports在线直播" Actions
- V体育ios版 - Actions
Substances
- "VSports手机版" Actions
- "VSports app下载" Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous (VSports在线直播)
