Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract
- PMID: 23960190
- PMCID: PMC3754860
- DOI: 10.1084/jem.20122762
Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract
Abstract
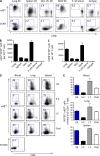
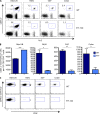
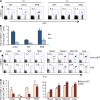
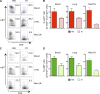
Developing efficacious vaccines against enteric diseases is a global challenge that requires a better understanding of cellular recruitment dynamics at the mucosal surfaces. The current paradigm of T cell homing to the gastrointestinal (GI) tract involves the induction of α4β7 and CCR9 by Peyer's patch and mesenteric lymph node (MLN) dendritic cells (DCs) in a retinoic acid-dependent manner VSports手机版. This paradigm, however, cannot be reconciled with reports of GI T cell responses after intranasal (i. n. ) delivery of antigens that do not directly target the GI lymphoid tissue. To explore alternative pathways of cellular migration, we have investigated the ability of DCs from mucosal and nonmucosal tissues to recruit lymphocytes to the GI tract. Unexpectedly, we found that lung DCs, like CD103(+) MLN DCs, up-regulate the gut-homing integrin α4β7 in vitro and in vivo, and induce T cell migration to the GI tract in vivo. Consistent with a role for this pathway in generating mucosal immune responses, lung DC targeting by i. n. immunization induced protective immunity against enteric challenge with a highly pathogenic strain of Salmonella. The present report demonstrates novel functional evidence of mucosal cross talk mediated by DCs, which has the potential to inform the design of novel vaccines against mucosal pathogens. .
Figures (V体育ios版)




V体育官网 - Comment in
-
"VSports手机版" Broadening the paradigm of mucosal dendritic cell-mediated induction of gut-homing on T cells.Gastroenterology. 2014 Mar;146(3):854-5. doi: 10.1053/j.gastro.2014.01.030. Epub 2014 Jan 24. Gastroenterology. 2014. PMID: 24468172 Free PMC article. No abstract available.
References
-
- Bhat P.V. 1998. Retinal dehydrogenase gene expression in stomach and small intestine of rats during postnatal development and in vitamin A deficiency. FEBS Lett. 426:260–262 10.1016/S0014-5793(98)00355-X - VSports最新版本 - DOI - PubMed
Publication types
MeSH terms
- Actions (VSports最新版本)
- VSports最新版本 - Actions
- VSports注册入口 - Actions
- V体育安卓版 - Actions
- "VSports app下载" Actions
- Actions (VSports)
- "V体育2025版" Actions
- "V体育官网入口" Actions
- "V体育ios版" Actions
- Actions (VSports)
- Actions (VSports)
- V体育官网入口 - Actions
- "VSports在线直播" Actions
- "V体育2025版" Actions
- "VSports注册入口" Actions
- V体育2025版 - Actions
- Actions (VSports最新版本)
- "VSports app下载" Actions
"VSports手机版" Substances
- V体育安卓版 - Actions
- "VSports手机版" Actions
- "V体育安卓版" Actions
- Actions (VSports app下载)
- "VSports最新版本" Actions
Grants and funding
LinkOut - more resources
Full Text Sources (VSports手机版)
"VSports在线直播" Other Literature Sources
Molecular Biology Databases
Research Materials

