Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes
- PMID: 23955712
- PMCID: PMC4050982
- DOI: V体育官网 - 10.1038/nm.3265
VSports注册入口 - Activation of the Nlrp3 inflammasome in infiltrating macrophages by endocannabinoids mediates beta cell loss in type 2 diabetes
V体育官网 - Abstract
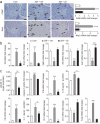
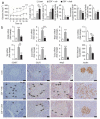
Type 2 diabetes mellitus (T2DM) progresses from compensated insulin resistance to beta cell failure resulting in uncompensated hyperglycemia, a process replicated in the Zucker diabetic fatty (ZDF) rat. The Nlrp3 inflammasome has been implicated in obesity-induced insulin resistance and beta cell failure VSports手机版. Endocannabinoids contribute to insulin resistance through activation of peripheral CB1 receptors (CB₁Rs) and also promote beta cell failure. Here we show that beta cell failure in adult ZDF rats is not associated with CB₁R signaling in beta cells, but rather in M1 macrophages infiltrating into pancreatic islets, and that this leads to activation of the Nlrp3-ASC inflammasome in the macrophages. These effects are replicated in vitro by incubating wild-type human or rodent macrophages, but not macrophages from CB₁R-deficient (Cnr1(-/-)) or Nlrp3(-/-) mice, with the endocannabinoid anandamide. Peripheral CB₁R blockade, in vivo depletion of macrophages or macrophage-specific knockdown of CB₁R reverses or prevents these changes and restores normoglycemia and glucose-induced insulin secretion. These findings implicate endocannabinoids and inflammasome activation in beta cell failure and identify macrophage-expressed CB₁R as a therapeutic target in T2DM. .
Figures
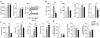
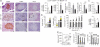

Comment in
-
Diabetes: Macrophages mediate β-cell loss in T2DM.Nat Rev Endocrinol. 2013 Nov;9(11):626. doi: 10.1038/nrendo.2013.177. Epub 2013 Sep 10. Nat Rev Endocrinol. 2013. PMID: 24019114 No abstract available.
References
-
- Ehses JA, et al. Increased number ot islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56:2356–2370. - PubMed
-
- Youm YH, et al. Elimination of the NLRP3-ASC inflammasorne protects against chronic obesity-induced pancreatic damage. Endocrinology. 2011;152:4039–4045. - V体育ios版 - PMC - PubMed
Publication types
- V体育ios版 - Actions
- "VSports注册入口" Actions
VSports在线直播 - MeSH terms
- "V体育官网" Actions
- "V体育安卓版" Actions
- Actions (V体育安卓版)
- "VSports" Actions
- Actions (VSports最新版本)
- Actions (V体育平台登录)
- "VSports最新版本" Actions
- V体育ios版 - Actions
- "VSports注册入口" Actions
- VSports最新版本 - Actions
- Actions (VSports注册入口)
- VSports手机版 - Actions
- "VSports app下载" Actions
- "VSports最新版本" Actions
- VSports注册入口 - Actions
- V体育官网 - Actions
- V体育ios版 - Actions
- "VSports" Actions
- Actions (V体育平台登录)
Substances
- Actions (VSports)
- V体育官网 - Actions
- Actions (V体育安卓版)
- V体育安卓版 - Actions
- "VSports手机版" Actions
- Actions (VSports最新版本)
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases (VSports app下载)
Miscellaneous

