The role of cyclase-associated protein in regulating actin filament dynamics - more than a monomer-sequestration factor
- PMID: 23908377
- PMCID: VSports最新版本 - PMC3730240
- DOI: 10.1242/jcs.128231 (VSports手机版)
The role of cyclase-associated protein in regulating actin filament dynamics - more than a monomer-sequestration factor
Abstract
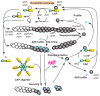
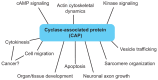
Dynamic reorganization of the actin cytoskeleton is fundamental to a number of cell biological events. A variety of actin-regulatory proteins modulate polymerization and depolymerization of actin and contribute to actin cytoskeletal reorganization. Cyclase-associated protein (CAP) is a conserved actin-monomer-binding protein that has been studied for over 20 years. Early studies have shown that CAP sequesters actin monomers; recent studies, however, have revealed more active roles of CAP in actin filament dynamics VSports手机版. CAP enhances the recharging of actin monomers with ATP antagonistically to ADF/cofilin, and also promotes the severing of actin filaments in cooperation with ADF/cofilin. Self-oligomerization and binding to other proteins regulate activities and localization of CAP. CAP has crucial roles in cell signaling, development, vesicle trafficking, cell migration and muscle sarcomere assembly. This Commentary discusses the recent advances in our understanding of the functions of CAP and its implications as an important regulator of actin cytoskeletal dynamics, which are involved in various cellular activities. .
Keywords: ADF/cofilin; Actin turnover; Cyclase-associated protein; Nucleotide exchange; Severing. V体育安卓版.
"V体育官网" Figures


References
-
- Agrawal P. B., Greenleaf R. S., Tomczak K. K., Lehtokari V. L., Wallgren-Pettersson C., Wallefeld W., Laing N. G., Darras B. T., Maciver S. K., Dormitzer P. R. et al.(2007). Nemaline myopathy with minicores caused by mutation of the CFL2 gene encoding the skeletal muscle actin-binding protein, cofilin-2. Am. J. Hum. Genet. 80, 162–167 10.1086/510402 - DOI (V体育安卓版) - PMC - PubMed
-
- Agrawal P. B., Joshi M., Savic T., Chen Z., Beggs A. H. (2012). Normal myofibrillar development followed by progressive sarcomeric disruption with actin accumulations in a mouse Cfl2 knockout demonstrates requirement of cofilin-2 for muscle maintenance. Hum. Mol. Genet. 21, 2341–2356 10.1093/hmg/dds053 - DOI (V体育平台登录) - PMC - PubMed
-
- Barrero R. A., Umeda M., Yamamura S., Uchimiya H. (2002). Arabidopsis CAP regulates the actin cytoskeleton necessary for plant cell elongation and division. Plant Cell 14, 149–163 10.1105/tpc.010301 - "V体育ios版" DOI - PMC - PubMed
Publication types
- "V体育官网" Actions
MeSH terms
- "V体育平台登录" Actions
- Actions (V体育官网)
- Actions (V体育安卓版)
- "V体育ios版" Actions
- "VSports注册入口" Actions
Substances
- V体育2025版 - Actions
- "VSports在线直播" Actions
- Actions (V体育平台登录)
"VSports" Grants and funding
LinkOut - more resources (V体育ios版)
Full Text Sources (V体育官网入口)
VSports注册入口 - Other Literature Sources
VSports手机版 - Research Materials
Miscellaneous

